Abstract
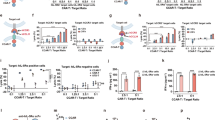
Chronic eosinophilic leukemia-not otherwise specified (CEL-NOS) is a rare, aggressive, fatal disease characterized by blood eosinophilia and dysfunction of organs infiltrated with eosinophils. Clinically, the disease manifests with weight loss, cough, weakness, diarrhea, and multi-organ dysfunction that is unresponsive to therapy. We developed a one-time gene therapy for CEL-NOS using an adeno-associated virus (AAV) expressing an anti-eosinophil monoclonal antibody (AAVrh.10mAnti-Eos) to provide sustained suppression of eosinophil numbers in blood, thus reducing eosinophil tissue invasion and organ dysfunction. A novel CEL-NOS model was developed in NOD-scid IL2rγnull (NSG) mice by administration of AAV expressing the cytokine IL5 (AAVrh.10mIL5), resulting in marked peripheral and tissue eosinophilia of the heart, lung, liver, and spleen, and eventually death. Mice were administered AAVrh.10mAnti-Eos (1011 genome copies) 4 wk after administration of AAVrh.10mIL5 and evaluated for anti-eosinophil antibody expression, blood eosinophil counts, organ eosinophil invasion, and survival. AAVrh.10mAnti-Eos expressed persistent levels of the anti-eosinophil antibody for >24 wk. Strikingly, CEL-NOS treated mice had markedly lower blood eosinophil levels and reduced mortality when compared with control treated mice. These results suggest that a single treatment with AAVrh.10mAnti-Eos has the potential to provide substantial therapeutic benefit to patients with CEL-NOS, a fatal malignant disorder.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gotlib J. World Health Organization-defined eosinophilic disorders: 2015 update on diagnosis, risk stratification, and management. Am J Hematol. 2015;90:1077–89.
Gotlib J. World Health Organization-defined eosinophilic disorders: 2017 update on diagnosis, risk stratification, and management. Am J Hematol. 2017;92:1243–59.
Reiter A, Gotlib J. Myeloid neoplasms with eosinophilia. Blood. 2017;129:704–14.
Crane MM, Chang CM, Kobayashi MG, Weller PF. Incidence of myeloproliferative hypereosinophilic syndrome in the United States and an estimate of all hypereosinophilic syndrome incidence. J Allergy Clin Immunol. 2010;126:179–81.
Dunphy CH. Chronic eosinophilic leukemia, not otherwise specified (CEL, NOS). Cancer Ther Rev. 2012;8:30–4.
Ogbogu PU, Bochner BS, Butterfield JH, Gleich GJ, Huss-Marp J, Kahn JE, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009;124:1319–25. e1313.
Helbig G, Soja A, Bartkowska-Chrobok A, Kyrcz-Krzemien S. Chronic eosinophilic leukemia-not otherwise specified has a poor prognosis with unresponsiveness to conventional treatment and high risk of acute transformation. Am J Hematol. 2012;87:643–5.
Part VII: Hematologic Malignancies. In: Hematology (Seventh Edition), edited by Hoffman R, Benz EJ, Silberstein LE, Heslop HE, Weitz JI, et al. Elsevier, 2018, 763–1443 pp.
Bochner BS. Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors. Clin Exp Allergy. 2009;39:317–24.
Song DJ, Cho JY, Lee SY, Miller M, Rosenthal P, Soroosh P, et al. Anti-Siglec-F antibody reduces allergen-induced eosinophilic inflammation and airway remodeling. J Immunol. 2009;183:5333–41.
Song DJ, Cho JY, Miller M, Strangman W, Zhang M, Varki A, et al. Anti-Siglec-F antibody inhibits oral egg allergen induced intestinal eosinophilic inflammation in a mouse model. Clin Immunol. 2009;131:157–69.
Zimmermann N, McBride ML, Yamada Y, Hudson SA, Jones C, Cromie KD, et al. Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy. 2008;63:1156–63.
Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101:5014–20.
Nutku E, Hudson SA, Bochner BS. Mechanism of Siglec-8-induced human eosinophil apoptosis: role of caspases and mitochondrial injury. Biochem Biophys Res Commun. 2005;336:918–24.
Varki A, Angata T. Siglecs–the major subfamily of I-type lectins. Glycobiology. 2006;16:1R–27R.
Nutku-Bilir E, Hudson SA, Bochner BS. Interleukin-5 priming of human eosinophils alters siglec-8 mediated apoptosis pathways. Am J Respir Cell Mol Biol. 2008;38:121–4.
Mao H, Kano G, Hudson SA, Brummet M, Zimmermann N, Zhu Z, et al. Mechanisms of Siglec-F-induced eosinophil apoptosis: a role for caspases but not for SHP-1, Src kinases, NADPH oxidase or reactive oxygen. PLoS One. 2013;8:e68143.
Camilleri AE, Nag S, Russo AR, Stiles KM, Crystal RG, Pagovich OE. Gene therapy for a murine model of eosinophilic esophagitis. Allergy. 2021;76:2740–52.
Mayginnes JP, Reed SE, Berg HG, Staley EM, Pintel DJ, Tullis GE. Quantitation of encapsidated recombinant adeno-associated virus DNA in crude cell lysates and tissue culture medium by quantitative, real-time PCR. J Virol Methods. 2006;137:193–204.
Wang F, Cui X, Wang M, Xiao W, Xu R. A reliable and feasible qPCR strategy for titrating AAV vectors. Med Sci Monit Basic Res. 2013;19:187–93.
Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–30.
Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–89.
Pagovich OE, Camilleri AE, Nag S, Stiles KM, Crystal RG. AAV-mediated anti-eosinophil gene therapy for eosinophilic esophagitis. Mol Ther. 2020;28:90.
Pagovich OE, Russo AR, Whaley AS, Cronin S, Matsumura Y, Stiles KM, et al. Gene Therapy for chronic eosinophilic esophagitis. Mol Ther. 2018;26:161.
Davidoff AM, Ng CY, Zhou J, Spence Y, Nathwani AC. Sex significantly influences transduction of murine liver by recombinant adeno-associated viral vectors through an androgen-dependent pathway. Blood. 2003;102:480–8.
De BP, Heguy A, Hackett NR, Ferris B, Leopold PL, Lee J, et al. High levels of persistent expression of alpha1-antitrypsin mediated by the nonhuman primate serotype rh.10 adeno-associated virus despite preexisting immunity to common human adeno-associated viruses. Mol Ther. 2006;13:67–76.
Nietupski JB, Hurlbut GD, Ziegler RJ, Chu Q, Hodges BL, Ashe KM, et al. Systemic administration of AAV8-alpha-galactosidase A induces humoral tolerance in nonhuman primates despite low hepatic expression. Mol Ther. 2011;19:1999–2011.
Wu Z, Sun J, Zhang T, Yin C, Yin F, Van Dyke T, et al. Optimization of self-complementary AAV vectors for liver-directed expression results in sustained correction of hemophilia B at low vector dose. Mol Ther. 2008;16:280–9.
Dane AP, Wowro SJ, Cunningham SC, Alexander IE. Comparison of gene transfer to the murine liver following intraperitoneal and intraportal delivery of hepatotropic AAV pseudo-serotypes. Gene Ther. 2013;20:460–4.
Morsia E, Reichard K, Pardanani A, Tefferi A, Gangat N. WHO defined chronic eosinophilic leukemia, not otherwise specified (CEL, NOS): a contemporary series from the Mayo Clinic. Am J Hematol 2020;95:E172–E174.
Curtis C, Ogbogu P. Hypereosinophilic syndrome. Clin Rev Allergy Immunol. 2016;50:240–51.
Falchi L, Verstovsek S. Eosinophilia in hematologic disorders. Immunol Allergy Clin North Am. 2015;35:439–52.
Gotlib J. World Health Organization-defined eosinophilic disorders: 2011 update on diagnosis, risk stratification, and management. Am J Hematol. 2011;86:677–88.
Strati P, Cortes J, Faderl S, Kantarjian H, Verstovsek S. Long-term follow-up of patients with hypereosinophilic syndrome treated with Alemtuzumab, an anti-CD52 antibody. Clin Lymphoma Myeloma Leuk. 2013;13:287–91.
Hudson SA, Herrmann H, Du J, Cox P, Haddad el B, Butler B, et al. Developmental, malignancy-related, and cross-species analysis of eosinophil, mast cell, and basophil siglec-8 expression. J Clin Immunol. 2011;31:1045–53.
Klion AD 55 - Eosinophilia. In: Keystone JS, Freedman DO, Kozarsky PE, Connor BA, Nothdurft HD (eds). Travel Medicine (Third Edition). Elsevier Inc.: London, 2013, pp. 501–9.
Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–66.
Dellon ES, Peterson KA, Murray JA, Falk GW, Gonsalves N, Chehade M, et al. Anti-Siglec-8 antibody for eosinophilic gastritis and duodenitis. N Engl J Med. 2020;383:1624–34.
Siebenhaar F, Bonnekoh H, Hawro T, Hawro MO, Michaelis EG, Rasmussen HS, et al. Safety and efficacy data of AK002, an anti-siglec-8 monoclonal antibody, in patients with indolent systemic mastocytosis (ISM): Results from a first-in-human, open-label phase 1 study. 2019; https://www.allakos.com/file.cfm/59/docs/Levine_at_al_AAAAI_March_2020.pdf.
Allakos. Overview. 2020; https://www.allakos.com/.
Altrichter S, Staubach P, Pasha M, Rasmussen H, Singh B, Chang A, et al. Clincial activity of AK002, an anti-siglec-8 antibody, in multiple forms of uncontrolled chronic urticaria. Ann Allergy Asthma Immunol. 2019;123:S27–S28.
Levine T, Tauber J, Nguyen Q, Anesi S, Chang P, Berdy G, et al. Clinical activity of AK002, an anti-siglec-8 antibody, in severe allergic conjunctivitis and comorbid atopic diseases. Ann Allergy, Asthma Immunol. 2019;123:S17.
Butt NM, Lambert J, Ali S, Beer PA, Cross NC, Duncombe A, et al. Guideline for the investigation and management of eosinophilia. Br J Haematol. 2017;176:553–72.
Valent P, Klion AD, Horny HP, Roufosse F, Gotlib J, Weller PF, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130:607–12. e609.
Rosenberg JB, De BP, Hicks MJ, Janda KD, Kaminsky SM, Worgall S, et al. Suppression of nicotine-induced pathophysiology by an adenovirus hexon-based antinicotine vaccine. Hum Gene Ther. 2013;24:595–603.
Celestin J, Frieri M. Eosinophilic disorders in various diseases. Curr Allergy Asthma Rep. 2012;12:18–24.
Mejia R, Nutman TB. Evaluation and differential diagnosis of marked, persistent eosinophilia. Semin Hematol. 2012;49:149–59.
Hastie E, Samulski RJ. Recombinant adeno-associated virus vectors in the treatment of rare diseases. Expert Opin Orphan Drugs. 2015;3:675–89.
Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12:341–55.
Franklin W, Goetzl EJ. Total absence of eosinophils in a patient with an allergic disorder. Ann Intern Med. 1981;94:352–3.
Abidi K, Belayachi J, Derras Y, Khayari ME, Dendane T, Madani N, et al. Eosinopenia, an early marker of increased mortality in critically ill medical patients. Intensive Care Med. 2011;37:1136–42.
Terradas R, Grau S, Blanch J, Riu M, Saballs P, Castells X, et al. Eosinophil count and neutrophil-lymphocyte count ratio as prognostic markers in patients with bacteremia: a retrospective cohort study. PLoS ONE. 2012;7:e42860.
Gleich GJ, Klion AD, Lee JJ, Weller PF. The consequences of not having eosinophils. Allergy. 2013;68:829–35.
Acknowledgements
We thank S Duan and J Paulson (Scripps Research Institute) for providing the sequence of the anti-Siglec-F antibody and N Mohamed for editorial assistance. These studies were supported, in part, by the Department of Genetic Medicine, Weill Cornell Medicine.
Author information
Authors and Affiliations
Contributions
ARR and SN: performed experimental work and data analysis/interpretation, reviewed manuscript for content. AC: performed experimental work and data analysis/interpretation, participated in the study design, prepared manuscript. RGC, OEP, and KMS: conceived study, performed data analysis/interpretation, prepared manuscript
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Pagovich, O.E., Stiles, K.M., Camilleri, A.E. et al. Gene therapy in a murine model of chronic eosinophilic leukemia-not otherwise specified (CEL-NOS). Leukemia 36, 525–531 (2022). https://doi.org/10.1038/s41375-021-01400-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-021-01400-4