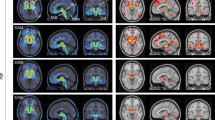
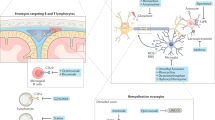
Abstract
Following the impressive progress in the treatment of relapsing–remitting multiple sclerosis (MS), the major challenge ahead is the development of treatments to prevent or delay the irreversible accumulation of clinical disability in progressive forms of the disease. The substrate of clinical progression is neuro-axonal degeneration, and a deep understanding of the mechanisms that underlie this process is a precondition for the development of therapies for progressive MS. PET imaging involves the use of radiolabelled compounds that bind to specific cellular and metabolic targets, thereby enabling direct in vivo measurement of several pathological processes. This approach can provide key insights into the clinical relevance of these processes and their chronological sequence during the disease course. In this Review, we focus on the contribution that PET is making to our understanding of extraneuronal and intraneuronal mechanisms that are involved in the pathogenesis of irreversible neuro-axonal damage in MS. We consider the major challenges with the use of PET in MS and the steps necessary to realize clinical benefits of the technique. In addition, we discuss the potential of emerging PET tracers and future applications of existing compounds to facilitate the identification of effective neuroprotective treatments for patients with MS.
Key points
-
PET enables direct in vivo measurement of key processes in the pathogenesis of neurodegeneration in multiple sclerosis (MS).
-
PET imaging of neuroinflammatory processes has shown that innate immune cell activation inside and outside visible lesions is a relevant pathogenic mechanism in MS, even in the earliest stages of the disease.
-
Novel PET tracers that specifically target innate immune cells, lymphocytes, metabolic pathways, endothelial molecules and active astrocytes could provide new insights into the role of inflammation in neurodegeneration in MS.
-
PET imaging of myelin in patients with MS has shown that myelin loss and failure of myelin repair can contribute to the accumulation of clinical disability.
-
PET imaging of the mitochondria and synaptic vesicles can be used to detect the earliest metabolic and structural changes in neurons in MS.
-
PET imaging of pathological processes could provide robust outcome measures in clinical trials of drugs designed to delay or prevent neurodegeneration in MS.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Thompson, A. & Ciccarelli, O. Towards treating progressive multiple sclerosis. Nat. Rev. Neurol. 16, 589–590 (2020).
Kawachi, I. & Lassmann, H. Neurodegeneration in multiple sclerosis and neuromyelitis optica. J. Neurol. Neurosurg. Psychiatry 88, 137–145 (2017).
McGinley, M. & Ontaneda, D. MS progression is predominantly driven by age-related mechanisms – NO. Mult. Scler. J. 25, 904–906 (2019).
Filippi, M. et al. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol. 18, 198–210 (2019).
Absinta, M., Lassmann, H. & Trapp, B. D. Mechanisms underlying progression in multiple sclerosis. Curr. Opin. Neurol. 33, 277–285 (2020).
Magliozzi, R. et al. A gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann. Neurol. 68, 477–493 (2010).
El Behi, M. et al. Adaptive human immunity drives remyelination in a mouse model of demyelination. Brain 140, 967–980 (2017).
Lloyd, A. F. & Miron, V. E. The pro-remyelination properties of microglia in the central nervous system. Nat. Rev. Neurol. 15, 447–458 (2019).
Hemmer, B., Kerschensteiner, M. & Korn, T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol. 14, 406–419 (2015).
Zrzavy, T. et al. Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain 140, 1900–1913 (2017). A neuropathological study that provides a comprehensive description of patterns of microglial activation in multiple sclerosis and demonstrates a significant reduction of ‘homeostatic’ microglia in the normal-appearing white matter as well as in active and slowly expanding lesions in the brain of patients with multiple sclerosis.
Jäckle, K. et al. Molecular signature of slowly expanding lesions in progressive multiple sclerosis. Brain 143, 2073–2088 (2020).
Starost, L. et al. Extrinsic immune cell-derived, but not intrinsic oligodendroglial factors contribute to oligodendroglial differentiation block in multiple sclerosis. Acta Neuropathol. 140, 715–736 (2020).
Absinta, M. et al. Association of chronic active multiple sclerosis lesions with disability in vivo. JAMA Neurol. 76, 1474 (2019).
Frischer, J. M. et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann. Neurol. 78, 710–721 (2015). This neuropathological paper describes how early active, late active, smouldering, inactive and shadow plaques are distributed across disease stages and disease forms in the brain, spinal cord and optic nerve of patients with multiple sclerosis.
Lassmann, H. Mechanisms of white matter damage in multiple sclerosis. Glia 62, 1816–1830 (2014).
van Wageningen, T. A. & van Dam, A-M. Much, if not all, of the cortical damage in MS can be attributed to the microglial cell – yes. Mult. Scler. J. 24, 895–896 (2018).
Mayo, L. et al. Regulation of astrocyte activation b n y glycolipids drives chronic CNS inflammation. Nat. Med. 20, 1147–1156 (2014).
Linnerbauer, M., Wheeler, M. A. & Quintana, F. J. Astrocyte crosstalk in CNS inflammation. Neuron 108, 608–622 (2020).
Singh, S. et al. Relationship of acute axonal damage, Wallerian degeneration, and clinical disability in multiple sclerosis. J. Neuroinflammation 14, 57 (2017).
Kornek, B. et al. Multiple sclerosis and chronic autoimmune encephalomyelitis. Am. J. Pathol. 157, 267–276 (2000).
Schultz, V. et al. Acutely damaged axons are remyelinated in multiple sclerosis and experimental models of demyelination. Glia 65, 1350–1360 (2017).
Patrikios, P. et al. Remyelination is extensive in a subset of multiple sclerosis patients. Brain 129, 3165–3172 (2006).
Mahad, D. H., Trapp, B. D. & Lassmann, H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 14, 183–193 (2015).
Campbell, G., Licht-Mayer, S. & Mahad, D. Targeting mitochondria to protect axons in progressive MS. Neurosci. Lett. 710, 134258 (2019).
Haider, L. et al. The topograpy of demyelination and neurodegeneration in the multiple sclerosis brain. Brain 139, 807–815 (2016).
Trapp, B. D. & Stys, P. K. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 8, 280–291 (2009). A review summarizing the evidence for the key role played by increased energy demand and reduced energy supply in inducing a chronic state of ‘virtual hypoxia’ in chronically demyelinated axons, which, if not reverted, leads to irreversible neuro-axonal degeneration in the brain of patients with multiple sclerosis.
Campbell, G. R. & Mahad, D. J. Mitochondrial changes associated with demyelination: consequences for axonal integrity. Mitochondrion 12, 173–179 (2012).
Boellaard, R. Standards for PET image acquisition and quantitative data analysis. J. Nucl. Med. 50, 11–21 (2009).
Wang, G., Rahmim, A. & Gunn, R. N. PET parametric imaging: past, present, and future. IEEE Trans. Radiat. Plasma Med. Sci. 4, 663–675 (2020).
Bertoldo, A., Rizzo, G. & Veronese, M. Deriving physiological information from PET images: from SUV to compartmental modelling. Clin. Transl. Imaging 2, 239–251 (2014).
National Institute of Mental Health. CNS radiotracer table. https://www.nimh.nih.gov/research/research-funded-by-nimh/therapeutics/cns-radiotracer-table.shtml.
Garden, G. A. & Campbell, B. M. Glial biomarkers in human central nervous system disease. Glia 64, 1755–1771 (2016).
Van De Bittner, G. C., Ricq, E. L. & Hooker, J. M. A philosophy for CNS radiotracer design. Acc. Chem. Res. 47, 3127–3134 (2014).
Pike, V. Considerations in the development of reversibly binding PET radioligands for brain imaging. Curr. Med. Chem. 23, 1818–1869 (2016).
Hooker, J. M. & Carson, R. E. Human positron emission tomography neuroimaging. Annu. Rev. Biomed. Eng. 21, 551–581 (2019).
Tonietto, M. et al. Plasma radiometabolite correction in dynamic PET studies: insights on the available modeling approaches. J. Cereb. Blood Flow. Metab. 36, 326–339 (2016).
Lyoo, C. H. et al. Cerebellum can serve as a pseudo-reference region in Alzheimer disease to detect neuroinflammation measured with PET radioligand binding to translocator protein. J. Nucl. Med. 56, 701–706 (2015).
Barletta, V. T. et al. Evidence of diffuse cerebellar neuroinflammation in multiple sclerosis by 11C-PBR28 MR-PET. Mult. Scler. 26, 668–678 (2020).
Turkheimer, F. E. et al. Reference and target region modeling of [11C]-(R)-PK11195 brain studies. J. Nucl. Med. 48, 158–167 (2007).
Schubert, J., Tonietto, M., Turkheimer, F., Zanotti-Fregonara, P. & Veronese, M. Supervised clustering for TSPO PET imaging. Eur. J. Nucl. Med. Mol. Imaging https://doi.org/10.1007/s00259-021-05309-z (2021).
Giannetti, P. et al. Increased PK11195-PET binding in normal-appearing white matter in clinically isolated syndrome. Brain 138, 110–119 (2015).
García-Lorenzo, D. et al. Validation of an automatic reference region extraction for the quantification of [18F]DPA-714 in dynamic brain PET studies. J. Cereb. Blood Flow. Metab. 38, 333–346 (2018).
Bodini, B. et al. Individual mapping of innate immune cell activation is a candidate marker of patient-specific trajectories of worsening disability in multiple sclerosis. J. Nucl. Med. 61, 1043–1049 (2020).
Veronese, M. et al. Quantification of [11C]PIB PET for imaging myelin in the human brain: a test-retest reproducibility study in high-resolution research tomography. J. Cereb. Blood Flow. Metab. 35, 1771–1782 (2015).
Bodini, B. et al. Dynamic imaging of individual remyelination profiles in multiple sclerosis. Ann. Neurol. 79, 726–738 (2016). This study was the first to use PET to measure myelin content changes in vivo in white matter lesions of patients with multiple sclerosis, showing that the potential of spontaneous myelin repair is heterogeneous across patients and is a key determinant in clinical disability.
Carotenuto, A. et al. [18F]Florbetapir PET/MR imaging to assess demyelination in multiple sclerosis. Eur. J. Nucl. Med. Mol. Imaging 47, 366–378 (2020).
Rissanen, E. et al. Automated reference region extraction and population-based input function for brain [11C]TMSX PET image analyses. J. Cereb. Blood Flow. Metab. 35, 157–165 (2015).
Salinas, C. A., Searle, G. E. & Gunn, R. N. The simplified reference tissue model: model assumption violations and their impact on binding potential. J. Cereb. Blood Flow. Metab. 35, 304–311 (2015).
Chen, Y. J. et al. Relative 11C-PiB delivery as a proxy of relative CBF: quantitative evaluation using single-session 15O-water and 11C-PiB PET. J. Nucl. Med. 56, 1199–1205 (2015).
Schubert, J. J. et al. Dynamic 11C-PiB PET shows cerebrospinal fluid flow alterations in Alzheimer disease and multiple sclerosis. J. Nucl. Med. 60, 1452–1460 (2019).
Huang, B., Law, M. W.-M. & Khong, P.-L. Whole-body PET/CT scanning: estimation of radiation dose and cancer risk. Radiology 251, 166–174 (2009).
Jiang, W., Chalich, Y. & Deen, M. J. Sensors for positron emission tomography applications. Sensors 19, 5019 (2019).
Pantel, A. R. et al. PennPET explorer: human imaging on a whole-body imager. J. Nucl. Med. 61, 144–151 (2020).
Catana, C. The dawn of a new era in low-dose PET imaging. Radiology 290, 657–658 (2019).
Chen, K. T. et al. Ultra-low-dose 18F-florbetaben amyloid PET imaging using deep learning with multi-contrast MRI inputs. Radiology 290, 649–656 (2019).
Banati, R. B. et al. The peripheral benzodiazepine binding site in the brain in multiple sclerosis. Brain 123, 2321–2337 (2000).
Nutma, E. et al. A quantitative neuropathological assessment of translocator protein expression in multiple sclerosis. Brain 142, 3440–3455 (2019). This study characterizes the cellular neuropathology associated with TSPO expression in multiple sclerosis, providing key insights for a correct interpretation of the results of TSPO PET studies in patients with this disease. It shows that, in multiple sclerosis, TSPO expression mainly arises from microglia, but a significant contribution also comes from astrocytes and, to a lesser extent, endothelial cells.
Matthews, P. M. Chronic inflammation in multiple sclerosis – seeing what was always there. Nat. Rev. Neurol. 15, 582–593 (2019). A detailed review summarizing the neuropathological features of chronic inflammation in multiple sclerosis and describing the MRI and PET techniques to measure this process in vivo in patients with this disease.
Singhal, T. et al. 18F-PBR06 versus 11C-PBR28 PET for assessing white matter translocator protein binding in multiple sclerosis. Clin. Nucl. Med. 43, e289–e295 (2018).
Singhal, T. et al. Gray matter microglial activation in relapsing vs progressive MS: A [F-18]PBR06-PET study. Neurol. Neuroimmunol. Neuroinflammation 6, e587 (2019).
Rissanen, E. et al. In vivo detection of diffuse inflammation in secondary progressive multiple sclerosis using PET imaging and the radioligand 11C-PK11195. J. Nucl. Med. 55, 939–944 (2014).
Politis, M. et al. Increased PK11195 PET binding in the cortex of patients with MS correlates with disability. Neurology 79, 523–530 (2012).
Hagens, M. H. J. et al. In vivo assessment of neuroinflammation in progressive multiple sclerosis: a proof of concept study with [18F]DPA714 PET. J. Neuroinflammation 15, 4–13 (2018).
Datta, G. et al. Neuroinflammation and its relationship to changes in brain volume and white matter lesions in multiple sclerosis. Brain 140, 2927–2938 (2017).
Sucksdorff, M. et al. Brain TSPO-PET predicts later disease progression independent of relapses in multiple sclerosis. Brain 143, 3318–3330 (2020). A convincing demonstration of the potential utility of TSPO PET, particularly for measurement of innate immune cell activation in the normal-appearing and perilesional white matter, to predict clinical progression in patients with multiple sclerosis.
Bezukladova, S. et al. Insights into disseminated MS brain pathology with multimodal diffusion tensor and PET imaging. Neurol. Neuroimmunol. Neuroinflammation 7, e691 (2020).
Dal-Bianco, A. et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7T magnetic resonance imaging. Acta Neuropathol. 133, 25–42 (2017).
Hametner, S. et al. The influence of brain iron and myelin on magnetic susceptibility and effective transverse relaxation – a biochemical and histological validation study. Neuroimage 179, 117–133 (2018).
Datta, G. et al. 11C-PBR28 and 18F-PBR111 detect white matter inflammatory heterogeneity in multiple sclerosis. J. Nucl. Med. 58, 1477–1482 (2017).
Giannetti, P. et al. Microglia activation in multiple sclerosis black holes predicts outcome in progressive patients: an in vivo [(11)C](R)-PK11195-PET pilot study. Neurobiol. Dis. 65, 203–210 (2014).
Poirion, E. et al. Structural and clinical correlates of a periventricular gradient of neuroinflammation in multiple sclerosis. Neurology 96, e1865–e1875 (2021). The first TSPO PET-based in vivo report of a periventricular gradient of neuroinflammation in patients with multiple sclerosis, which is associated with microstructural damage and disability progression.
Sucksdorff, M. et al. Evaluation of the effect of fingolimod treatment on microglial activation using serial PET imaging in multiple sclerosis. J. Nucl. Med. 58, 1646–1651 (2017).
Sucksdorff, M. et al. Natalizumab treatment reduces microglial activation in the white matter of the MS brain. Neurol. Neuroimmunol. Neuroinflammation 6, e574 (2019).
Guilarte, T. R. TSPO in diverse CNS pathologies and psychiatric disease: a critical review and a way forward. Pharmacol. Ther. 194, 44–58 (2019).
Kreisl, W. C. et al. PET imaging of neuroinflammation in neurological disorders. Lancet Neurol. 19, 940–950 (2020).
Owen, D. R. et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J. Cereb. Blood Flow. Metab. 32, 1–5 (2012).
Ikawa, M. et al. 11C-ER176, a radioligand for 18-kDa translocator protein, has adequate sensitivity to robustly image all three affinity genotypes in human brain. J. Nucl. Med. 58, 320–325 (2017).
Owen, D. R. et al. Pro-inflammatory activation of primary microglia and macrophages increases 18kDa translocator protein expression in rodents but not humans. J. Cereb. Blood Flow. Metab. 37, 2679–2690 (2017).
Nutma, E. et al. Activated microglia do not increase 18kDa translocator protein (TSPO) expression in the multiple sclerosis brain. Glia https://doi.org/10.1002/glia.24052 (2021).
Bonsack, F., Alleyne, C. H. & Sukumari-Ramesh, S. Augmented expression of TSPO after intracerebral hemorrhage: a role in inflammation? J. Neuroinflammation 13, 151 (2016).
Meyer, J. H. et al. Neuroinflammation in psychiatric disorders: PET imaging and promising new targets. Lancet Psychiatry 7, 1064–1074 (2020).
Narayanaswami, V. et al. Emerging PET radiotracers and targets for imaging of neuroinflammation in neurodegenerative diseases: outlook beyond TSPO. Mol. Imaging 17, 1536012118792317 (2018).
Jain, P. et al. Neuroinflammation PET imaging: current opinion and future directions. J. Nucl. Med. 61, 1107–1112 (2020).
Ghadery, C., Best, L. A., Pavese, N., Tai, Y. F. & Strafella, A. P. PET evaluation of microglial activation in non-neurodegenerative brain diseases. Curr. Neurol. Neurosci. Rep. 19, 38 (2019).
Horti, A. G. et al. PET imaging of microglia by targeting macrophage colony-stimulating factor 1 receptor (CSF1R). Proc. Natl Acad. Sci. USA 116, 1686–1691 (2019).
Beaino, W. et al. Purinergic receptors P2Y12R and P2X7R: potential targets for PET imaging of microglia phenotypes in multiple sclerosis. J. Neuroinflammation 14, 259 (2017).
Villa, A. et al. Identification of new molecular targets for PET imaging of the microglial anti-inflammatory activation state. Theranostics 8, 5400–5418 (2018).
James, M. L. et al. Imaging B cells in a mouse model of multiple sclerosis using 64Cu-rituximab PET. J. Nucl. Med. 58, 1845–1851 (2017).
Syvänen, S. & Eriksson, J. Advances in PET imaging of P-glycoprotein function at the blood-brain barrier. ACS Chem. Neurosci. 4, 225–237 (2013).
Nahrendorf, M. et al. 18F-4V for PET-CT imaging of VCAM-1 expression in atherosclerosis. JACC Cardiovasc. Imaging 2, 1213–1222 (2009).
Montalban, X. et al. Placebo-controlled trial of an oral BTK inhibitor in multiple sclerosis. N. Engl. J. Med. 380, 2406–2417 (2019).
Waniewski, R. A. & Martin, D. L. Preferential utilization of acetate by astrocytes is attributable to transport. J. Neurosci. 18, 5225–5233 (1998).
Kato, H. et al. Astrocyte metabolism in multiple sclerosis investigated by 1-C-11 acetate PET. J. Cereb. Blood Flow. Metab. 41, 369–379 (2020).
Takata, K. et al. 11C-acetate PET imaging in patients with multiple sclerosis. PLoS ONE 9, e111598 (2014).
Levitt, P., Pintar, J. E. & Breakefield, X. O. Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proc. Natl Acad. Sci. USA 79, 6385–6389 (1982).
Saura, J. et al. Increased monoamine oxidase B activity in plaque-associated astrocytes of Alzheimer brains revealed by quantitative enzyme radioautography. Neuroscience 62, 15–30 (1994).
Ng, K. P. et al. Monoamine oxidase B inhibitor, selegiline, reduces 18F-THK5351 uptake in the human brain. Alzheimers. Res. Ther. 9, 25 (2017).
Ishibashi, K., Miura, Y., Hirata, K., Toyohara, J. & Ishii, K. 18F-THK5351 PET can identify astrogliosis in multiple sclerosis plaques. Clin. Nucl. Med. 45, e98–e100 (2020).
Ishibashi, K., Kameyama, M., Miura, Y., Toyohara, J. & Ishii, K. Head-to-head comparison of the two MAO-B radioligands, 18F-THK5351 and 11C-L-deprenyl, to visualize astrogliosis in patients with neurological disorders. Clin. Nucl. Med. 46, e31–e33 (2021).
Vuorimaa, A. et al. Increased [11C]TMSX binding to A2A receptors around MS plaques and the normal appearing white matter in secondary progressive multiple sclerosis is explained by astrocytic A2A expression. ECTRIMS Online Library https://onlinelibrary.ectrims-congress.eu/ectrims/2018/ectrims-2018/228945/anna.vuorimaa.increased.5B11c5Dtmsx.binding.to.a2a.receptors.around.ms.plaques.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D1%2Asearch%3Dvuorimaa (2018).
Rissanen, E. et al. Adenosine A2A receptors in secondary progressive multiple sclerosis: a [(11)C]TMSX brain PET study. J. Cereb. Blood Flow. Metab. 33, 1394–1401 (2013).
Tyacke, R. J. et al. Evaluation of 11C-BU99008, a PET ligand for the imidazoline2 binding site in human brain. J. Nucl. Med. 59, 1597–1602 (2018).
Wilson, H. et al. Imidazoline 2 binding sites reflecting astroglia pathology in Parkinson’s disease: an in vivo 11C-BU99008 PET study. Brain 142, 3116–3128 (2019).
Escartin, C. et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 24, 312–325 (2021).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017).
van der Weijden, C. W. J. et al. Myelin quantification with MRI: a systematic review of accuracy and reproducibility. Neuroimage 226, 117561 (2021).
Mancini, M. et al. An interactive meta-analysis of MRI biomarkers of myelin. eLife 9, e61523 (2020). This excellent interactive meta-analysis reviews all published validation studies on quantitative MRI measures of myelin, concluding that the highest correlations with myelin content are found for magnetization transfer and relaxometry-based measures.
Petiet, A. et al. Ultrahigh field imaging of myelin disease models: toward specific markers of myelin integrity? J. Comp. Neurol. 527, 2179–2189 (2019).
Stankoff, B. et al. Imaging of CNS myelin by positron-emission tomography. Proc. Natl Acad. Sci. USA 103, 9304–9309 (2006).
Wu, C. et al. A novel PET marker for in vivo quantification of myelination. Bioorg. Med. Chem. 18, 8592–8599 (2010).
Wu, C. et al. Longitudinal positron emission tomography imaging for monitoring myelin repair in the spinal cord. Ann. Neurol. 74, 688–698 (2013).
Bajaj, A. et al. Identification of the protein target of myelin-binding ligands by immunohistochemistry and biochemical analyses. J. Histochem. Cytochem. 61, 19–30 (2013).
Stankoff, B., Poirion, E., Tonietto, M. & Bodini, B. Exploring the heterogeneity of MS lesions using positron emission tomography: a reappraisal of their contribution to disability. Brain Pathol. 28, 723–734 (2018).
Stankoff, B. et al. Imaging central nervous system myelin by positron emission tomography in multiple sclerosis using [methyl-11C]-2-(4-methylaminophenyl)-6-hydroxybenzothiazole. Ann. Neurol. 69, 673–680 (2011).
De Paula Faria, D. et al. PET imaging of focal demyelination and remyelination in a rat model of multiple sclerosis: comparison of [11C]MeDAS, [11C]CIC and [11C]PIB. Eur. J. Nucl. Med. Mol. Imaging 41, 995–1003 (2014).
Tiwari, A. D. et al. Novel 18F-labeled radioligands for positron emission tomography imaging of myelination in the central nervous system. J. Med. Chem. 62, 4902–4914 (2019).
Wu, C. et al. Discovery of 1,2,3-triazole derivatives for multimodality PET/CT/cryoimaging of myelination in the central nervous system. J. Med. Chem. 60, 987–999 (2017).
Wang, C., Wu, C., Zhu, J., Miller, R. H. & Wang, Y. Design, synthesis, and evaluation of coumarin-based molecular probes for imaging of myelination. J. Med. Chem. 54, 2331–2340 (2011).
Auvity, S. et al. Repurposing radiotracers for myelin imaging: a study comparing 18F-florbetaben, 18F-florbetapir, 18F-flutemetamol, 11C-MeDAS, and 11C-PiB. Eur. J. Nucl. Med. Mol. Imaging 47, 490–501 (2020).
Matías-Guiu, J. J. A. J. et al. Amyloid PET imaging in multiple sclerosis: an 18F-florbetaben study. BMC Neurol. 15, 243 (2015).
Zeydan, B. et al. Pittsburgh compound-B PET white matter imaging and cognitive function in late multiple sclerosis. Mult. Scler. J. 24, 739–749 (2018).
Carvalho, R. H. F. et al. [11C]PIB PET imaging can detect white and grey matter demyelination in a non-human primate model of progressive multiple sclerosis. Mult. Scler. Relat. Disord. 35, 108–115 (2019).
Pytel, V. et al. Amyloid PET findings in multiple sclerosis are associated with cognitive decline at 18 months. Mult. Scler. Relat. Disord. 39, 101926 (2020).
Fisher, E. et al. Imaging correlates of axonal swelling in chronic multiple sclerosis brains. Ann. Neurol. 62, 219–228 (2007).
Trapp, B. D. et al. Cortical neuronal densities and cerebral white matter demyelination in multiple sclerosis: a retrospective study. Lancet Neurol. 17, 870–884 (2018).
Lubetzki, C., Zalc, B., Williams, A., Stadelmann, C. & Stankoff, B. Remyelination in multiple sclerosis: from basic science to clinical translation. Lancet Neurol. 19, 678–688 (2020). A detailed review providing an up-to-date overview of the process of remyelination in multiple sclerosis, including evidence from human neuropathology, experimental models and human imaging studies, and reporting on the progress in the development of therapeutic strategies to promote myelin repair in patients with this disease.
Pietroboni, A. M. et al. Amyloid PET as a marker of normal-appearing white matter early damage in multiple sclerosis: correlation with CSF β-amyloid levels and brain volumes. Eur. J. Nucl. Med. Mol. Imaging 46, 280–287 (2019).
Brugarolas, P. et al. Development of a PET radioligand for potassium channels to image CNS demyelination. Sci. Rep. 8, 607 (2018).
Rodríguez-Rangel, S., Bravin, A. D., Ramos-Torres, K. M., Brugarolas, P. & Sánchez-Rodríguez, J. E. Structure-activity relationship studies of four novel 4-aminopyridine K+ channel blockers. Sci. Rep. 10, 52 (2020).
Wei, W. et al. Predicting PET-derived demyelination from multimodal MRI using sketcher-refiner adversarial training for multiple sclerosis. Med. Image Anal. 58, 101546 (2019).
Wei, W. et al. Predicting PET-derived myelin content from multisequence MRI for individual longitudinal analysis in multiple sclerosis. Neuroimage 223, 117308 (2020).
Roelcke, U. et al. Reduced glucose metabolism in the frontal cortex and basal ganglia of multiple sclerosis patients with fatigue: a 18F-fluorodeoxyglucose positron emission tomography study. Neurology 48, 1566–1571 (1997).
Blinkenberg, M. et al. Cortical cerebral metabolism correlates with MRI lesion load and cognitive dysfunction in MS. Neurology 54, 558–564 (2000).
Radu, C. G., Shu, C. J., Shelly, S. M., Phelps, M. E. & Witte, O. N. Positron emission tomography with computed tomography imaging of neuroinflammation in experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 104, 1937–1942 (2007).
Buck, D. et al. 18F-FDG PET detects inflammatory infiltrates in spinal cord experimental autoimmune encephalomyelitis lesions. J. Nucl. Med. 53, 1269–1276 (2012).
Freeman, L. et al. The neuronal component of gray matter damage in multiple sclerosis: a [(11)C]flumazenil positron emission tomography study. Ann. Neurol. 78, 554–567 (2015).
Kang, Y. et al. A multi-ligand imaging study exploring GABAergic receptor expression and inflammation in multiple sclerosis. Mol. Imaging Biol. 22, 1600–1608 (2020).
Mansur, A. et al. Characterization of 3 PET tracers for quantification of mitochondrial and synaptic function in healthy human brain: 18F-BCPP-EF, 11C-SA-4503, and 11C-UCB-J. J. Nucl. Med. 61, 96–103 (2020). A pilot study involving healthy controls that identifies the optimal kinetic analysis method and the suitable acquisition duration for three PET radioligands that target mitochondria and synaptic function, 18F-BCPP-EF, 11C-SA-4503 and 11C-UCB-J. The results of this study are essential for the application of these tracers to the exploration of mitochondrial damage and synaptic dysfunction in neurodegenerative diseases, including multiple sclerosis.
Finnema, S. J. et al. Imaging synaptic density in the living human brain. Sci. Transl. Med. 8, 348ra96 (2016).
Chen, M.-K. et al. Assessing synaptic density in Alzheimer disease with synaptic vesicle glycoprotein 2A positron emission tomographic imaging. JAMA Neurol. 75, 1215–1224 (2018).
Matuskey, D. et al. Synaptic changes in Parkinson disease assessed with in vivo imaging. Ann. Neurol. 87, 329–338 (2020).
Finnema, S. J. et al. Reduced synaptic vesicle protein 2A binding in temporal lobe epilepsy: a [11C]UCB-J positron emission tomography study. Epilepsia 61, 2183–2193 (2020).
Holmes, S. E. et al. Lower synaptic density is associated with depression severity and network alterations. Nat. Commun. 10, 1529 (2019).
Onwordi, E. C. et al. Synaptic density marker SV2A is reduced in schizophrenia patients and unaffected by antipsychotics in rats. Nat. Commun. 11, 246 (2020).
Mansur, A. et al. Test–retest variability and reference region-based quantification of 18F-BCPP-EF for imaging mitochondrial complex I in the human brain. J. Cereb. Blood Flow. Metab. 41, 771–779 (2021).
Dangond, F. et al. Facing the urgency of therapies for progressive MS – a progressive MS Alliance proposal. Nat. Rev. Neurol. 17, 185–192 (2021). This article illustrates why the International Progressive MS Alliance has chosen to develop a new funding programme for experimental medicine trials which have the potential to advance therapies targeting neurodegeneration in multiple sclerosis.
Acknowledgements
The studies discussed that were performed in the Paris Brain Institute received funding from: ANR (Agence Nationale de la Recherche; grant MNP2008-007125); CEA (Commissariat aux Energies Atomiques); European Committee for Treatment and Research in MS (ECTRIMS); ELA (European Leukodystrophy Association; grant 2007-0481); ARSEP Foundation (Fondation pour l’aide à la recherche sur la sclérose en plaques); FRM (Fondation pour la Recherche Médicale); INSERM-DHOS (grant 2008–recherche clinique et translationnelle); Investissements d’avenir (grant ANR-10-IAIHU-06); JNLF (Journées de Neurologie de Langue Française); Programme Hospitalier de Recherche Clinique (PHRC national, 2010; APHP). APHP (Assistance Publique des Hôpitaux de Paris) sponsored these clinical studies. The authors are grateful to the Bouvet-Labruyère family for their constant support of our research projects. The authors thank the members of the CIC (Clinical Investigation Center; C. Louapre, J.C. Corvol) and CENIR (Centre de NeuroImagerie de l’ICM; S. Lehericy, E. Bardinet) in the Paris Brain Institute, and members of the SHFJ (Service Hospitalier Frédéric Joliot, Commissariat aux Energies atomiques; M. Bottlaender, B. Kunhast, P. Gervais, V. Lebon) for their invaluable contribution to the authors’ clinical studies and for their technical support. We also thank B. Dubois and the Scientific Committee of the INSIGHT study for kindly providing the 18F-florbetapir PET image. The authors are grateful to C. Fumat and C. Théry for their contribution to the conception and the realization of Fig. 1.
Author information
Authors and Affiliations
Contributions
All authors contributed to all aspects of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Neurology thanks F. Barkhof and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bodini, B., Tonietto, M., Airas, L. et al. Positron emission tomography in multiple sclerosis — straight to the target. Nat Rev Neurol 17, 663–675 (2021). https://doi.org/10.1038/s41582-021-00537-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-021-00537-1