Abstract
Background
Previous study reports that fibroblast growth factor 21 (FGF21) could ameliorate hepatic fibrosis, but its mechanisms have not been fully investigated.
Methods and results
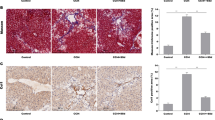
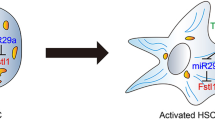
In this study, three models were used to investigate the mechanism by which FGF21 alleviates liver fibrosis. Hepatic fibrosis animal models were respectively induced by CCL4 and dimethylnitrosamine. Our results demonstrated that liver index and liver function were deteriorated in both models. Hematoxylin and eosin and Masson’s staining showed that the damaged tissue architectonics were observed in the mice of both models. Treatment with FGF21 significantly ameliorated these changes. ELISA analysis showed that the serum levels of IL-1β, IL-6 and TNF-α were significantly elevated in both models. However, administration of FGF21 significantly reduced these inflammatory cytokines. Real-time PCR and Western blot analysis showed that treatment with FGF21 significantly decreased mRNA and protein expressions of collagenI, α-SMA and TGF-β. Platelet-derived growth factor-BB (PDGF-BB) stimulant was used to establish the experimental cell model in hepatic stellate cells (HSCs). Real-time PCR and Western blot analysis demonstrated that the expression of collagenI and α-SMA were significantly upregulated by this stimulant in model group. Interestingly, our results showed that mRNA and protein expressions of leptin were also significantly induced in PDGF-BB treated HSCs. Administration of FGF21 significantly reduced leptin expression in a dose dependent manner and these effects were reversed in siRNA (against β-klotho) transfected HSCs. Furthermore, the leptin signaling pathways related protein p-ERK/t-ERK, p-STAT3/STAT3 and TGF-β were significantly downregulated by FGF21 treatment in a dose dependent manner. The expressions of SOCS3 and Nrf-2 were enhanced by treatment with FGF21. The underlying mechanism may be that FGF21 regulates leptin-STAT3 axis via Nrf-2 and SOCS3 pathway in activated HSCs.
Conclusions
FGF21 ameliorates hepatic fibrosis by multiple mechanisms.




Similar content being viewed by others
Data availability
The data analyzed during the current study may be available upon reasonable request.
References
Kharitonenkov A, Adams AC (2014) Inventing new medicines: the FGF21 story. Mol Metab 3(3):221–229
Nobuyuki, Itoh. FGF21 as a Hepatokine, Adipokine, and Myokine in Metabolism and Diseases. Frontiers in Endocrinology. 5(2):p.152–159, (2014).
Dutchak PA, Katafuchi T, Bookout AL et al (2012) Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell 148:556–567
Berglund ED, Li CY, Bina HA et al (2009) Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology. 150(9):4084–4093
Lin Z, Pan X, Wu F et al (2015) Fibroblast growth factor 21 prevents atherosclerosis by suppression of hepatic sterol regulatory element-binding protein-2 and induction of adiponectin in mice. Circulation 131(21):1861–1871
Li S, Zhu Z, Xue M et al (1865) (2019) Fibroblast growth factor 21 protects the heart from angiotensin II-induced cardiac hypertrophy and dysfunction via SIRT1. Biochimica et Biophysica Acta (BBA) 6:1241–1252
Pan X, Shao Y, Wu F et al (2018) FGF21 Prevents angiotensin II-Induced hypertension and vascular dysfunction by activation of ACE2/angiotensin-(1–7) axis in mice. Cell Metab 27(6):1323–1337
Lin X, Li G, He X et al (2014) FGF21 inhibits apolipoprotein(a) expression in HepG2 cells via the FGFR1-ERK1/2-Elk-1 pathway. Mol Cell Biochem 393(1–2):33–42
Xu P, Zhang Y, Wang W et al (2015) Long-term administration of fibroblast growth factor 21 prevents chemically-induced hepatocarcinogenesis in mice. Digest Dis Sci 60:3032–3043
Opoku YK et al (2020) Fibroblast growth factor-21 ameliorates hepatic encephalopathy by activating the stat3-socs3 pathway to inhibit activated hepatic stellate cells. EXCLJ J 19:567–581
Xu P, Zhang Y, Wang W (2016) Fibroblast growth factor 21 attenuates hepatic fibrogenesis through TGF-β/smad2/3 and NF-κB signaling pathways. Toxicol Appl Pharmacol 290:43–53
Lee KJ, Jang YO, Cha SK et al (2018) Expression of fibroblast growth factor 21 and β-klotho regulates hepatic fibrosis through the nuclear factor-κB and c-Jun N-terminal kinase pathways. Gut Liver 12(4):449–456
Aydin MM, Akcali KC (2018) Liver fibrosis. Turk J Gastroenterol 29(1):14–21
Bataller R, Brenner DA (2005) Liver fibrosis. J Clin Investig 115(2):209–218
Ramachandran P, Iredale JP (2012) Liver fibrosis: a bidirectional model of fibrogenesis and resolution. QJM 105(9):813–817
Tsuchida T, Friedman SL (2017) Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol 14(7):397–411
Zhang C et al (2016) Liver fibrosis and hepatic stellate cells: etiology, pathological hallmarks and therapeutic targets. World J Gastroenterol 22(48):10512
Saxena NK et al (2004) Leptin as a novel profibrogenic cytokine in hepatic stellate cells: mitogenesis and inhibition of apoptosis mediated by extracellular regulated kinase (Erk) and Akt phosphorylation. FASEB J 18(13):1612–1614
Kouta I, Mrzljak A, Kolari B et al (2020) Leptin as a key player in insulin resistance of liver cirrhosis? A cross-sectional study in liver transplant candidates. J Clin Med 9(2):560
Chen Z, Jain A, Liu H et al (2019) Targeted drug delivery to hepatic stellate cells for the treatment of liver fibrosis. J Pharmacol Exp Ther 370(3):695–702
Yanguas SC, Cogliati B, Eillebrods J (2016) Experimental models of liver fibrosis. Arch Toxicol 90(5):1025–1048
Scholten D, Trebicka J, Liedtke C et al (2015) The carbon tetrachloride model in mice. Lab Anim 49(1):4–11
Korsrud GO, Harold C et al (1972) Sensitivity of several serum enzymes in detecting carbon tetrachloride-induced liver damage in rats. Toxicol Appl Pharmacol 22:474–483
Verna L, Whysner J, Williams GM (1996) N-Nitrosodiethylamine mechanistic data and risk assessment: bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol Ther 71:57–81
Liu X, Dai R, Ke M et al (2017) Differential proteomic analysis of dimethylnitrosamine (DMN)-induced liver fibrosis. Proteomics 17(22):1700267
George J, Tsuchishima M, Tsutsumi M (2019) Molecular mechanisms in the pathogenesis of N-nitrosodimethylamine induced hepatic fibrosis. Cell Death Dis 10(1):52–59
Van Dijk F et al (2015) Targeted therapies in liver fibrosis: combining the best parts of platelet-derived growth factor BB and interferon gamma. Front Med 2:257–260
Friedman SL (2008) Mechanisms of hepatic fibrogenesis. Gastroenterology 134(6):1655–1669
Friedman SL, Yamasaki G, Wong L (1994) Modulation of transforming growth factor beta receptors of rat lipocytes during the hepatic wound healing response. J Biol Chem 269(14):10551–10558
Borkham-Kamphorst E, Weiskirchen R (2016) The PDGF system and its antagonists in liver fibrosis. Cytokine Growth Factor Rev 28:53–61
Bethanis SK, Theocharis SE (2006) Leptin in the field of hepatic fibrosis: a pivotal or an incidental player? Dig Dis Sci 51(10):1685–1696
Kwon O, Kim KW, Kim M (2016) Leptin signaling pathways in hypothalamic neurons. Cell Mol Life Sci 73(7):1457–1477
Sahin GS, Dhar M, Dillon C (2019) leptin stimulates synaptogenesis in hippocapal nerons via KLF4 and SOCS3 inhibition of STAT3 signaling. Mol Cell Neurosci 20:123–128
Howard JK, Flier JS (2006) Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol Metab 17(9):365–370
Kim GY, Jeong H, Yoon H-M (2020) Anti-inflammatory mechanisms of suppressors of cytokine signaling target ROS via NRF-2/thioredoxin induction and inflammasome activation in macrophages. BMB Rep 53(12):640–645
Zhang S et al (2018) FGF21 attenuates pulmonary fibrogenesis through ameliorating oxidative stress in vivo and in vitro. Biomed Pharmacother 103:1516–1525
Jang JH, Kang KJ, Kim YH et al (2008) Reevaluation of experimental model of hepatic fibrosis induced by hepatotoxic drugs: an easy, applicable, and reproducible model. Transpl Proc 40(8):2700–2703
Yang X, Lechleider RJ, Chen L et al (1999) Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-beta. EMBO J 18(5):1280–1291
Trombley S, Maugars G, Kling P et al (2012) Effects of long-term restricted feeding on plasma leptin, hepatic leptin expression and leptin receptor expression in juvenile Atlantic salmon (Salmo salar L.). Gen Comp Endocrinol 175(3):92–99
Otte C, Otte JM et al (2004) Expression of leptin and leptin receptor during the development of liver fibrosis and cirrhosis. Exp Clin Endocrinol Diabetes 112(01):10–17
Foglia B, Cannito S, Bocca C et al (2019) ERK pathway in activated, myofibroblast-like, hepatic stellate cells: a critical signaling crossroad sustaining liver fibrosis. Int J Mol Sci 20(11):2700
Lu C et al (2017) Nrf2 induces lipocyte phenotype via a SOCS3-dependent negative feedback loop on JAK2/STAT3 signaling in hepatic stellate cells. Int Immunopharmacol 49:203–211
Gong Z, Lin J, Zheng J et al (2019) Dahuang Zhechong pill attenuates CCl4-induced rat liver fibrosis via the PI3K-Akt signaling pathway. J Cell Biochem 121(2):1431–1440
Funding
This study was supported by a grant from the major prophase project of Heilongjiang development and reform commission ([2011]1570), education department of Heilongjiang province (TSTAU-R2018017) and National Key R&D program of China (2017YFD0501102, 2017YFD0501103-03).
Author information
Authors and Affiliations
Contributions
FRM performed the research, and analyzed the data. KMH, KK, QH, YKC and XHJ participated in data collection and analysis. WX and DSL contributed to the initial and consequent project discussion, manuscript discussion and revision. All the authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflict of interest.
Ethical approval
This study was approved by the ethics committee of Northeast Agriculture University. All experimental protocols followed the guidelines issued by National Institute of Health and the Institutional Animal Care and Use Committee of Northeast Agriculture University. The mice were euthanatized under anesthesia induced by intraperitoneal injection of 1.2% avertin (Sigma, USA) at a dose of 20 μL/g body weight.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Meng, F., Khoso, M.H., Kang, K. et al. FGF21 ameliorates hepatic fibrosis by multiple mechanisms. Mol Biol Rep 48, 7153–7163 (2021). https://doi.org/10.1007/s11033-021-06707-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06707-0