Abstract
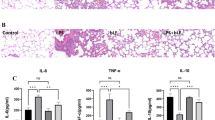
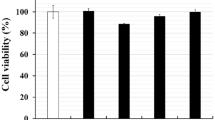
In this study, the regulation effects of selenium (Se) on the expression of pyrin domain-containing protein (NLRP) 3 inflammasome and reactive oxygen species (ROS) in bovine mammary epithelial cells (bMECs) infected by Staphylococcus aureus (S. aureus) were detected. bMECs were treated with 8 μmol/L Na2SeO3 for 12 h before infection with S. aureus for 2 h. Through flow cytometry, Western blot, and qRT-PCR analysis, the expression of ROS and NLRP3 imflammasome was detected. Results showed Se significantly reduced the ROS level in bMECs; at the same time, the expressions of NLRP3, ASC, caspase-1, Pro-IL-1β, and IL-1β were also decreased. In conclusion, Se inhibits S. aureus-induced inflammation by suppressing the activation of NLRP3 inflammasome and ROS in bMECs.



Similar content being viewed by others
Data Availability
All data used during the study appear in the submitted article.
References
Abebe R, Hatiya H, Abera M et al (2016) Bovine mastitis: prevalence, risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed, South Ethiopia. BMC Vet Res 12(1):270
Pumipuntu N, Kulpeanprasit S, Santajit S et al (2017) Screening method for Staphylococcus aureus identification in subclinical bovine mastitis from dairy farms. Vet World 10:721–726
Sowash MG, Uhlemann AC (2014) Community-associated methicillin-resistant Staphylococcus aureus case studies. Methods Mol Biol 1085:25–69
Jeong YJ, Kang MJ, Lee SJ et al (2015) NOD2 and RIP2 contribute to innate immune responses in mouse neutrophils. Cytokine 143:269–276
Philpott DJ, Girardin SE (2004) The role of toll-like receptors and Nod proteins in bacterial infection. Mol Immunol 41:1099–1108
Philpott DJ, Sorbara MT, Robertson SJ et al (2014) NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol 14:9–23
Wang H, Bi CL, Wang Y et al (2018) Selenium ameliorates Staphylococcus aureus-induced inflammation in bovine mammary epithelial cells by inhibiting activation of TLR2, NF-κB and MAPK signaling pathways. BMC Vet Res 14(1):197
Girardin SE, Travassos LH, Herve M et al (2003) Peptidoglycan molecular requirements allowing detection by NOD1 and NOD2. J Biol Chem 278(43):41702–41708
Melehani JH, James D, Dumont AL et al (2015) Staphylococcus aureus Leukocidin A/B (LukAB) kills human monocytes via host NLRP3 and ASC when extracellular, but not intracellular. Plos Pathog. 11(6):e1004970
Zhao XB, Pu DB, Zhao ZZ et al (2017) Teuvincenone F suppresses LPS-induced inflammation and NLRP3 Inflammasome activation by attenuating NEMO ubiquitination. Front Pharmacol 8:565
Duewell P, Kono H, Rayner KJ et al (2010) NLRP3 inflammasome are required for atherogenesis and activated by cholesterol crystals. Nature 464(7306):1357–1361
Lee DJ, Du F, Chen SW et al (2015) Regulation and function of the caspase-1 in an inflammatory microenvironment. J Invest Dermatol 135(8):2012–2020
Li YF, Nanayakkara G, Sun Y et al (2017) Analyses of caspase-1-regulated transcriptomes in various tissues lead to identification of novel IL-1β-, IL-8- and sirtuin-1-independent pathway. J Hematol Oncol 10:40
Abais JM, Xia M, Zhang Y et al (2015) Redox regulation of NLRP3 inflammasomes: ROS as Trigger or Effector? Antioxid Redox Signal 22(13):1111–1129
Kebaier C, Chamberland RR, Allen IC et al (2012) Staphylococcus aureus alpha-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J Infect Dis 205:807–817
Smith AD, Cheung L, Beshah E et al (2013) Selenium status alters the immune response and expulsion of adult Heligmosomoides bakeri worms in mice. Infect Immun 81:2546–2553
Mehdi Y, Durfrasne I (2016) Selenium in cattle: a review. Molecules 21(3):545
Hosnedlova B, Kepinska M, Skalickova S et al (2017) A summary of new findings on the biological effects of selenium in selected animal species-a critical review. Int J Mol Sci 18(10):2209
Yang H, Fang J, Jia X et al (2011) Chemopreventive effects of early-stage and late-stage supplementation of vitamin E and selenium on esophageal carcinogenesis in rats maintained on a low vitamin E/selenium diet. Carcinogenesis 32:381–388
Hu XQ, Song R, Zhang LB (2019) Effect of oxidative stress on the estrogen-NOS-NO-KCa channel pathway in uteroplacental dysfunction: its implication in pregnancy complications. Oxid Med Cell Longev 2019:9194269
Kondoh M, Ohga N, Akiyama K et al (2013) Hypoxia-induced reactive oxygen species cause chromosomal abnormalities in endothelial cells in the tumor microenvironment. PLoS One. 8(11):e80349
Benko S, Philpott DJ, Girardin SE et al (2008) The microbial and danger signals that activate Nod-like receptors. Cytokine 43(3):368–373
Ma JK, Zhu S, Guo YF et al (2019) Selenium attenuates Staphylococcus aureus mastitis in mice by inhibiting the activation of NLRP3 inflammasome and NF-κB/MAPK pathway. Biol Trace Elem Res 191:159–166
Kelly N, Jeltema D, Duan YH et al (2019) The NLRP3 Inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci 20(13):3328
Dinarello CA (2009) Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27:519–550
Ichinohe T, Lee HK, Ogura Y et al (2009) Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med 206(1):79–87
Muruve DA, Pétrilli V, Zaiss AK et al (2008) The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452(7183):103–107
Ma MW, Wang J, Dhandapani KM et al (2017) NADPH oxidase 2 regulates NLRP3 inflammasome activation in the brain after traumatic brain injury. Oxid Med Cell Longev 2017:6057609
Funding
This work was supported by the National Natural Science Foundation (NO. 31802254) of China; the Science and Technology Project of Shandong Province higher education institutions (NO. J18KB074); and the Key Research and Development Project of Hebei (19226625D).
Author information
Authors and Affiliations
Contributions
Yan Yang and Zhennan Wang contributed to the overall study design and supervised all research. Junjun Liu analyzed the data and prepared figures and contributed partly to writing and finally revising the manuscript and data analysis. Shenjin Lv drafted and revised the first version of the manuscript. All the authors reviewed and finally approved the manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
All experimental procedures were conducted with the approval of the Institutional Animal Care and Use Committee of Linyi University.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, Y., Lv, S., Wang, Z. et al. Selenium Ameliorates S. aureus-Induced Inflammation in Bovine Mammary Epithelial Cells by Regulating ROS-Induced NLRP3 Inflammasome. Biol Trace Elem Res 200, 3171–3175 (2022). https://doi.org/10.1007/s12011-021-02924-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02924-7