Abstract
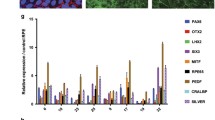
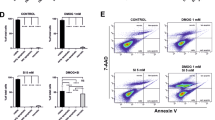
Aging, chronic oxidative stress, and inflammation are major pathogenic factors in the development and progression of age-related macular degeneration (AMD) with the loss of retinal pigment epithelium (RPE). The human RPE contains a subpopulation of progenitors (i.e., RPE stem cells—RPESCs) whose role in the RPE homeostasis is under investigation. We evaluated the paracrine effects of mature RPE cells exposed to oxidative stress (H2O2) on RPESCs behavior through co-cultural, morphofunctional, and bioinformatic approaches. RPESCs showed a decline in proliferation, an increase of the senescence-associated β-galactosidase activity, the acquisition of a senescent-like secretory phenotype (SASP), and the reduction of their stemness and differentiation competencies. IL-6 and Superoxide Dismutase 2 (SOD2) seem to be key molecules in RPESCs response to oxidative stress. Our results get insight into stress-induced senescent-associated molecular mechanisms implicated in AMD pathogenesis. The presence of chronic oxidative stress in the microenvironment reduces the RPESCs abilities, inducing and/or maintaining a pro-inflammatory retinal milieu that in turn could affect AMD onset and progression.






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- AMD:
-
Age-related macular degeneration
- CRALBP:
-
Cellular retinaldehyde-binding protein
- COX-2:
-
Cyclooxygenase 2
- DMEM:
-
Dulbecco's modified eagle medium
- FBS:
-
Fetal bovine serum
- GA:
-
Geographic atrophy
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- H-RPE:
-
Human retinal pigment epithelial cells
- H2O2:
-
Hydrogen peroxide
- KLF4:
-
Kruppel-like factor 4
- MSCs:
-
Mesenchymal stromal cells
- MITF:
-
Microphthalmia-associated transcription factor
- OTX2:
-
Orthodenticle homeobox 2
- PAX6:
-
Paired box 6
- PBS:
-
Phosphate-buffered saline
- MEM:
-
Minimum essential medium eagle
- PEDF:
-
Pigment-epithelium derived factor
- RtEGM:
-
Retinal pigment epithelial cell growth medium
- RPESCs:
-
Retinal pigment epithelial progenitor cells
- RPE:
-
Retinal pigment epithelium
- RPE65:
-
Retinal pigment epithelium-specific 65 kDa protein
- SASP:
-
Senescence-associated secretory phenotype
- SA-β-Gal:
-
Senescence-associated β-galactosidase
- SOX2:
-
SRY-box transcription factor 2
- SOD-2:
-
Superoxide dismutase 2
References
Al-Zamil WM, Yassin SA (2017) Recent developments in age-related macular degeneration: a review. Clin Interv Aging 12:1313–1330. https://doi.org/10.2147/cia.s143508
Wong WL, Su X, Li X et al (2014) Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2:e106-116. https://doi.org/10.1016/s2214-109x(13)70145-1
Bird AC, Bressler NM, Bressler SB et al (1995) An international classification and grading system for age-related maculopathy and age-related macular degeneration. The international ARM epidemiological study group. Surv Ophthalmol 39:367–374. https://doi.org/10.1016/s0039-6257(05)80092-x
Garcia-Layana A, Cabrera-Lopez F, Garcia-Arumi J et al (2017) Early and intermediate age-related macular degeneration: update and clinical review. Clin Interv Aging 12:1579–1587. https://doi.org/10.2147/cia.s142685
Wang S, Wang X, Cheng Y et al (2019) Autophagy dysfunction, cellular senescence, and abnormal immune-inflammatory responses in AMD: from mechanisms to therapeutic potential. Oxid Med Cell Longev 2019:3632169. https://doi.org/10.1155/2019/3632169
Lee JS (2019) Cellular senescence, aging, and age-related disease: special issue of BMB reports in 2019. BMB Rep 52:1–2
Kinnunen K, Petrovski G, Moe MC, Berta A, Kaarniranta K (2012) Molecular mechanisms of retinal pigment epithelium damage and development of age-related macular degeneration. Acta Ophthalmol 90:299–309. https://doi.org/10.1111/j.1755-3768.2011.02179.x
Gupta T, Saini N, Arora J, Sahni D (2017) Age-related changes in the chorioretinal junction: an immunohistochemical study. J Histochem Cytochem 65:567–577. https://doi.org/10.1369/0022155417726507
Blasiak J (2020) Senescence in the pathogenesis of age-related macular degeneration. Cell Mol Life Sci 77:789–805. https://doi.org/10.1007/s00018-019-03420-x
Glotin AL, Debacq-Chainiaux F, Brossas JY et al (2008) Prematurely senescent ARPE-19 cells display features of age-related macular degeneration. Free Radic Biol Med 44:1348–1361. https://doi.org/10.1016/j.freeradbiomed.2007.12.023
Marazita MC, Dugour A, Marquioni-Ramella MD, Figueroa JM, Suburo M (2016) Oxidative stress-induced premature senescence dysregulates VEGF and CFH expression in retinal pigment epithelial cells: implications for age-related macular degeneration. Redox Biol 7:78–87. https://doi.org/10.1016/j.redox.2015.11.011
Yu AL, Fuchshofer R, Kook D, Kampik A, Bloemendal H, Welge-Lussen U (2009) Subtoxic oxidative stress induces senescence in retinal pigment epithelial cells via TGF-beta release. Invest Ophthalmol Vis Sci 50:926–935. https://doi.org/10.1167/iovs.07-1003
Mariotti C, Lazzarini R, Nicolai M et al (2015) Comparative study between amniotic-fluid mesenchymal stem cells and retinal pigmented epithelium (RPE) stem cells ability to differentiate towards RPE cells. Cell Tissue Res 362:21–3. https://doi.org/10.1007/s00441-015-2185-9
Salero E, Blenkinsop TA, Corneo B et al (2012) Adult human RPE can be activated into a multipotent stem cell that produces mesenchymal derivatives. Cell Stem Cell 10:88–95. https://doi.org/10.1016/j.stem.2011.11.018
Stanzel BV, Liu Z, Somboonthanakij S et al (2014) Human RPE stem cells grown into polarized RPE monolayers on a polyester matrix are maintained after grafting into rabbit subretinal space. Stem Cell Rep 2:64–77. https://doi.org/10.1016/j.stemcr.2013.11.005
Davis RJ, Alam NM, Zhao C et al (2017) The developmental stage of adult human stem cell-derived retinal pigment epithelium cells influences transplant efficacy for vision rescue. Stem Cell Rep 9:42–49. https://doi.org/10.1016/j.stemcr.2017.05.016
Schwartz SD, Regillo CD, Lam BL et al (2015) Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 385:509–516. https://doi.org/10.1016/s0140-6736(14)61376-3
Lazzarini R, Nicolai M, Pirani V, Mariotti C, Di Primio R (2018) Effects of senescent secretory phenotype acquisition on human retinal pigment epithelial stem cells. Aging (Albany NY) 10:3173–3184. https://doi.org/10.18632/aging.101624
Jassal B, Matthews L, Viteri G et al (2020) The reactome pathway knowledgebase. Nucleic Acids Res 48:D498–D503. https://doi.org/10.1093/nar/gkz1031
Jacob KD, Noren Hooten N, Trzeciak AR, Evans MK (2013) Markers of oxidant stress that are clinically relevant in aging and age-related disease. Mech Ageing Dev 134:139–157. https://doi.org/10.1016/j.mad.2013.02.008
Kauppinen A, Paterno JJ, Blasiak J, Salminen A, Kaarniranta K (2016) Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci 73:1765–1786. https://doi.org/10.1007/s00018-016-2147-8
Boulton M, Dayhaw-Barker P (2001) The role of the retinal pigment epithelium: topographical variation and ageing changes. Eye (London) 15:384–389. https://doi.org/10.1038/eye.2001.141
Harris J, Subhi Y, Sorensen TL (2017) Effect of aging and lifestyle on photoreceptors and retinal pigment epithelium: cross-sectional study in a healthy Danish population. Pathobiol Aging Age Relat Dis 7:1398016. https://doi.org/10.1080/20010001.2017.1398016
Sarna T, Burke JM, Korytowski W et al (2003) Loss of melanin from human RPE with aging: possible role of melanin photooxidation. Exp Eye Res 76:89–98. https://doi.org/10.1016/s0014-4835(02)00247-6
Ardeljan D, Chan CC (2013) Aging is not a disease: distinguishing age-related macular degeneration from aging. Prog Retin Eye Res 37:68–89. https://doi.org/10.1016/j.preteyeres.2013.07.003
Stanton CM, Wright AF (2014) Inflammatory biomarkers for AMD. Adv Exp Med Biol 801:251–257. https://doi.org/10.1007/978-1-4614-3209-8_32
Abokyi S, To CH, Lam TT, Tse DY (2020) Central role of oxidative stress in age-related macular degeneration: evidence from a review of the molecular mechanisms and animal models. Oxid Med Cell Longev. https://doi.org/10.1155/2020/7901270
Ung L, Pattamatta U, Carnt N, Wilkinson-Berka JL, Liew G, White AJR (2017) Oxidative stress and reactive oxygen species: a review of their role in ocular disease. Clin Sci (London) 131:2865–2883. https://doi.org/10.1042/cs20171246
Tower J (2012) Stress and stem cells. Wiley Interdiscip Rev Dev Biol. 1(6):789–802. https://doi.org/10.1002/wdev.56
Kourtis N, Tavernarakis N (2011) Cellular stress response pathways and ageing: intricate molecular relationships. EMBO J 30(13):2520–2531. https://doi.org/10.1038/emboj.2011.162
Kültz D (2005) Molecular and evolutionary basis of the cellularstress response. Annu Rev Physiol 67:225–267. https://doi.org/10.1146/annurev.physiol.67.040403.103635
Kook D, Wolf AH, Yu AL et al (2008) The protective effect of quercetin against oxidative stress in the human RPE in vitro. Invest Ophthalmol Vis Sci 49:1712–1720. https://doi.org/10.1167/iovs.07-0477
Meyer P, Maity P, Burkovski A et al (2017) A model of the onset of the senescence associated secretory phenotype after DNA damage induced senescence. PLoS Comput Biol 13:e1005741. https://doi.org/10.1371/journal.pcbi.1005741
Yang SR, Park JR, Kang KS (2015) Reactive oxygen species in mesenchymal stem cell aging: implication to lung diseases. Oxid Med Cell Longev 2015:486263. https://doi.org/10.1155/2015/486263
Bharti K, Nguyen MT, Skuntz S, Bertuzzi S, Arnheiter H (2006) The other pigment cell: specification and development of the pigmented epithelium of the vertebrate eye. Pigment Cell Res 19:380–394. https://doi.org/10.1111/j.1600-0749.2006.00318.x
Funding
This study was supported by a Grant of Università Politecnica delle Marche to Monica Mattioli-Belmonte.
Author information
Authors and Affiliations
Contributions
Conceptualization: RL, MN, CM, and MMB; methodology and investigation: RL (cell culture, RT-PCR, gene analysis) and GL (Morphological investigation); writing—review and editing: all authors; supervision and funding acquisition: CM, MB, and MMB.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no competing interests associated with the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lazzarini, R., Nicolai, M., Lucarini, G. et al. Oxidative stress in retinal pigment epithelium impairs stem cells: a vicious cycle in age-related macular degeneration. Mol Cell Biochem 477, 67–77 (2022). https://doi.org/10.1007/s11010-021-04258-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-021-04258-3