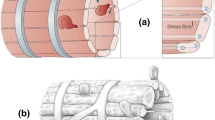
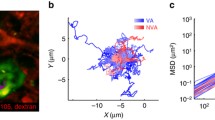
Abstract
The flow patterns of red blood cells through the spleen are intimately linked to clearance of senescent RBCs, with clearance principally occurring within the open flow through the red pulp and slits of the venous sinus system that exists in humans, rats, and dogs. Passage through interendothelial slits (IESs) of the sinus has been shown by MacDonald et al. (Microvasc Res 33:118–134, 1987) to be mediated by the caliber, i.e., slit opening width, of these slits. IES caliber within a given slit of a given sinus section has been shown to operate in an asynchronous manner. Here, we describe a model and simulation results that demonstrate how the supporting forces exerted on the sinus by the reticular meshwork of the red pulp, combined with asymmetrical contractility of stress fibers within the endothelial cells comprising the sinus, describe this vital and intriguing behavior. These results shed light on the function of the sinus slits in species such as humans, rats, and dogs that possess sinusoidal sinuses. Instead of assuming a passive mechanical filtering mechanism of the IESs, our proposed model provides a mechanically consistent explanation for the dynamically modulated IES opening/filtering mechanism observed in vivo. The overall perspective provided is also consistent with the view that IES passage serves as a self-protective mechanism in RBC vesiculation and inclusion removal.









Similar content being viewed by others
References
Abraldes JG, Sarlieve P, Tandon P (2014) Measurement of portal pressure. Clin Liver Dis 18:779–792
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002) The Cell. Garland Science
Allarg A, Schiffelers RM, van Solinge WW, van Wijk R (2013) Red blood cell vesiculation in herditaryhemolytic anemia. Front Physiol 4:1–15
Asaro RJ, Lubarda VA (2006) Mechanics of solids and materials. Cambridge Univ Press, New York
Asaro RJ, Zhu Q (2020) Vital Erythrocyte phenomena: what can theory, modeling, and simulation offer? Biomech Model Mechanobiol. https://doi.org/10.1007/s10237-020-01302-x
Asaro RJ, Zhu Q, Cabrales P (2018) Erythrocyte Aging, Protection via Vesiculation: an analysis methodology via oscillatory flow. Front Physiol 9:1607
Asaro RJ, Zhu Q, MacDonald IC (2020) Tethering, evagination, and vesiculation via cell-cell interactions in microvascular flow. Biomech Model Mechanobiol 20:31–53
Atkinson M, Sherlock S (1954) Intraspleenic pressure as index of portal venous pressure. Lancet 1954:1325–1327
Bauer K, Kratzer M, Otte M, de Quintana KL, Hagmann J, Arnold GJ, Eckerskorn C, Lottspeich F, Siess W (2000) Blood 96:4236–4245
Biosfleury A, Mohandas N (1977) Anti-body-induced spherocytic anemia II. Splenic passage and sequestration of red cells. Blood Cells 3:197–208
Blue J, Weiss L (1981) Electron microscopy of the red pulp of the dog spleen including vascular arrangements, periarterial macrophage sheaths (ellipsoids), and the contractile, innervated reticular meshwork. Am J Anat 161:189–218
Bonomini M, Sirolli V, Gizza F, Di Stante S, Grilli A, Felaco M (2002) Enhanced adhesion of human uremic eruthrocytes to vascular endothelium: role of phosphatidylserine exposure. Kidney Int 62:1358–1363
Bosman GJ, Willekens FL, Werre JM (2005) Erythrocyte aging: a more than superficial resemblance to apoptosis? Cell Physiol Biochem 16(1–3):1–8
Bosman G, Willekens FLA, Weere JM (2012) Erythocyte Senesecence. In: Land F, Föller M (eds) Erythrocytes, physiology and pathophysiology. Imperial College Press, London
Carlson BM (2018) The human body: linking structure and function. Academic Press, New York
Cesta MF (2006) Normal structure, function, and histology of the spleen. Taxicol Pathol 34:455–465
Chen LT, Weiss L (1972) Electron microscopy of the red pulp of human spleen. Am J Anat 134:425–458
Chen LT, Weiss L (1973) The role of the sinus wall in the passage of erythrocytes through the spleen. Blood 41:529–537
Cho Y, De Bruyn PPH (1975) Passage of red blood cells through the sinusoidal wall of the spleen. Am J Anat 142:91–106
Ciana A, Achilli C, Gaur A, Minetti G (2017a) Membrane remodeling and vesicle formation during ageing of human red blood cells. Cell Physiol Biochem 42:1127–1138
Ciana A, Achilli C, Minetti G (2017b) Spectrin and other membrane-skeletal components in human red blood cells of different age. Cell Physiol Biochem 42:1139–1152
Cinar E, Zhou S, DeCourcey J, Wang Y, Waugh RE, Wan J (2015) Peizo1 regulates mechanotransductive release of ATP from human RBCs. PNAS 112:11783–11788
Closse C, Dochary-Prigent J, Boisseau MR (1999) Phosphatidylserine-related adhesion of human erythrocytes to vascular endothelium. Br J Haematol 107:1365–2141
Dietrich HH, Ellsworth ML, Sorague RS, Dacey RG (2000) Red blood cell regulation of microvascular tone through adenosine triphosphate. Am J Physiol Heart Circul Physiol 278(2):H1294–H1298
Diez-Silva M, Dao M, Han J, Lim C-T, Suresh S (2010) Shape and biomechanical characteristics of human red blood cells in health and disease. MRS Bull 35:382–388
Diez-Silva M, Park Y, Huang S, Bow H, Mercereau-Puijalon O et al (2012) Pf155/RESA protein influences the dynamic microcirculatory behavior of ring-stage Plasmodium falciparum infected red blood cells. Sci Rep 2:614–620
Drenckhahn D, Wagner J (1986) Stress fibers in the splenic sinus endothelium in situ: molecular structure, relationship to extracellular matrix, and contractility. J Cell Biol 102:1738–1747
Ellsworth ML, Forrester T, Ellis CG, Dietrich HH (1995) The erythrocyte as a regulator of vascular tone. Am J Physiol 269(6–2):H2155
Ellsworth ML, Ellis CG, Sprague RS (2016) Role of erythrocyte-released ATP in the regulation of microvascular oxygen supply in skeletal muscle. Acta Physiol 216(3):265–276
Essler M, Retzer M, Bauer M, Heemskerk JW, Aepfelbacher M, Siess W (1999) Mildly oxidized low density lipoprotein induces contraction of human endothelial cells through activation of Rho/Rho kinase and inhibition of myosin light chain phosphatase. J Biol Chem 274(43):30361–30364
Fens MHAM, van Eijk R, Andringa G, van Rooijen KL, Dijstelbloem HM, Rasmussen JT, de Vooght KMK, Schiffelers RM, Gaillard CAJM, van Solinge WW (2012) A role for activated endothelial cells in red blood cell clearance: implications for vasopathology. Haematologica 97(4):500–508
Franke RP, Grate M, Schnitter H, Seiffge D, Mittermayer C (1984) Induction of human vascular endothelial stress fibers by fluid shear stress. Nature 307:648–649
Fujita T (1974) A scanning electron microscope study of the human spleen. Arch Histol Jap 37:187–216
Gálfiová P, Varga I, Kopáni M, Michalka P, Michalová J, Jakubovský J, Polák S (2009) Some possibilities of representing microcirculation in human spleen. Biologia 64(6):1242–1246
Gottlieb Y, Topez O, Cohen LA, Yakov LD, Haber T, Morgenstern A, Weiss A, Berman KC, Meyron-Holtz E (2012) Physiologically aged red blood cells undergo erythrophagocytosis in vivo but not in vitro. Haematologica 97(7):994–1002
Groom AC, MacDonald IC, Schmidt EE (2002) Splenic microcirculatory blood flow and function with respect to red blood cells. In: Bowdler AJ (ed) The complete speen. Humanna Press, Totowa, pp 23–50
Hataba Y, Kirino Y, Suzuki T (1981) Scanning electron microscopic study of the red pulp of mouse spleen. J Electr Microsc 30:46–56
Henry B, Roussel C, Carucci M, Brousse V, Ndour PA, Buffet P (2020) The human spleen in malaria: filter of shelter? Trends Parasitol 36:435–446
Huang S, Undisz A, Diez-Silva M, Bow H, Dao M, Han J (2013) Dynamic deformability of Plasmodium falciparum-infected erythrocytes exposed to artesunate in vitro. Integrat Biol 5(2):414–422
Kang I, Panneerselvam D, Panoskaltis VP, Eppel SJ, Marchant RE, Doerchuk CM (2008) Changes in the hyperelastic properties of endothelial cells induced by tumor necrosis factot-\(\alpha\). Biophys J 94:3273–3285
Kataoka N, Iwaki K, Hashimoto K, Mochizuki S, Ogasawara Y, Sato M, Tsujioka K, Kajiya F (2008) Measurements of endothelial cell-to-cell and cell-to-substrate gaps and micromechanical properties of endothelial cells during monocyte adhesion. PNAS 99:15638–15643
Klei TRL, de Back DZ, Asif PJ, Verkuijlen PJJH, Veldthuis M, Ligthart PC, Berghuis J, Clifford E, Beuger BM, van den Berg TK, van Zwieten R, El Nemar W, van Bruggen R (2018) Glycophorin-C sialylation regulates Lu/BCAM adhesive capacity during erythrocyte againg. Blood Adv 2:14–24
Klei TRL, Dalimot J, Nota B, Veldthuis M, Mul FPJ, Rademakers T, Hoogenboezem M, Nagelkerke SQ, van IJcken WFJ, Oole E, Scendsen P, Moestrup SK, van Alphen FPJ, Meijer AB, Kuijpers TW, van Zwieten R, van Bruggen R (2020) Hemolysis in the spleen drives erythreocyte turnover. Blood 136(14):1579–1589
Koyama S, Aoki S, Deguchi K (1964) Electron microscopic observation of the splenic red pulp with special reference to the pitting function. Mie Med J 14:143–188
Leal JKF, Adjobo-Hermans MJW, Bosman GJCM (2018) Red blood cell homeostasis: mechanisms and effects of microvesicle generation in health and disease. Front Physiol 9:703
Lee W-C, Russell B, Rénia L (2019) Sticking for a cause: the falciparum malaria parasites cytoadherence paradigm. Front Immunol 10:01444
Levesque MJ, Groom AC (1976) Washout kinetics of red cells and plasma from the spleen. Am J Physiol 231:1665–1671
Li H, Lu L, Li X, Buffet PA, Dao M, Karniadakis GE, Suresh S (2018) Mechanics of diseased red blood cells in human spleen and consequences for hereditary blood disorders. Proc Natl Acad Sci USA 115(38):9574–9579
Lim YB, Thingna J, Kong F, Dao M, Cao J, Lim C-T (2020) Temperature-induced catch-slip to slip bond transit in plasmodium falciparum-infected erythrocytes. Biophys J 118(1):105–116
Liu SC, Zhai S, Palek J, Golan DE, Amato D et al (1990) Molecular defect of the band 3 protein in Southeast Asian Ovalocytosis. J N Engl Med
Liu SC, Palek J, Yi SJ, Nichols PE, Derick LH et al (1995) Molecular basis of alterewd red blood cell membrane properties in Southeast Asian Ovalocytosis: role of the mutant band 3 protein in band 3 oligomerization and retention by the membrane skeleton. Blood 86:349–358
Low PS, Waugh SM, Zinke K (1985) The role of hemoglobin denaturation and band 3 clustering in red cell aging. Science 227:531–533
Lutz HU (2004) Innate immune and non-immune mediators of erythrocyte clearance. Cell Mol Biol (Noisy-le-grand) 50(2):107–116
Lutz HU, Bogdanova A (2013) Mechanisms tagging senescent red blood cells for clearance in healthy humans. Front Physiol 4:1–15
MacDonald IC, Ragan DM, Schmidt EE, Groom AC (1987) Kinetics of red blood cell passage through interendothelial slits into venous sinuses in rat spleen, analyzed by in vivo microscopy. Microvasc Res 33:118–134 (See also videos on youtube.com)
MacDonald IC, Schmidt EE, Groom AC (1991) The high splenic hematocrit: a rheological consequence of red cell flow through the reticular meshwork. Micrvasc Res 42:60–76
Mancuso JE, Jayaraman A, Ristenpart WD (2018) Centrifugation-induced release of ATP from red blood cells. PLOS 13(9):e0203270
Marginedas-Freixa I, Alvarez CL, Moras M, Leal Denis MF, Hattab C et al (2018) Human erythrocytes release ATP by a novel pathway involving VDAC oligomerization independent of pannexin-1. Sci Rep 8:11384
Mebius R, Kraal G (2005) Structure and function of the spleen. Nat Rev Immunol 5:606–616
Mills JP, Diez-Silva M, Quinn DJ, Dao M, Lang MJ, Tan KSW, Lim C-T, Milon G, David PH, Mercereau-Puijalon O, Bonnefoy S, Suresh S (2007) Effect of plasmodial RESA protein on deformability of human red blood cells harboring Plasmodium falciparum. Proc Natl Acad Sci USA 104(22):9213–9217
Miyoshi M, Fujita T (1971) Stero-fine structure of the splenic red pulp. A combined scanning and transmission electron microscope study on dog and rat spleen. Arch Histol Jap 33:225–246
Mohandas N, Gallagher PG (2008) Red cell membrane: past, present, and future. Blood 112:3939–3948
Mohanty JG, Nagababu E, Rifkind JM (2014) Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell againg. Front Physiol 5:1–6
Nehls V, Drenckhahn D (1991) Demonstration of actin filament stress fibers in microvascular endothelial cells in situ. Microvasc Res 42:103–112
Opdyke DF (1993) Hemodynamics of blood flow though the spleen. Am J Physiol 219(1):102–106
Opdyke DF, Apostolico R (1966) Splenic contraction and optical density of blood. Am J Physiol 211:329–334
Pandey KB, Rizvi SI (2010) Markers of oxidative stress in erythrocytes and plasma during aging in humans. Oxid Med Cell Longev 3(1):2–12
Pandey KB, Rizvi SI (2011) Biomarkers of oxidative stress in red blood cells. Biomed Pap Med Fac Univ Palacky Czeh Rep 155(2):131–136
Pernow J, Mahdi A, Yang J, Zhou Z (2019) Red blood cell dysfunction: a new player in cardiovascular disease. Cardiovasc Res 115:1596–1605
Pivkin IV, Peng Z, Karniadakis GE, Buffet PA, Dao M, Suresh S (2016) Biomechanics of red blood cells in human spleen and consequences for physiology and disease. Proc Natl Acad Sci USA 113(28):7804–7809
Price AK, Fisher DJ, Martin RS, Spence DM (2004) Deformation-induced release of ATP from erythrocytes in a poly(dimethylsiloxane)-based microchip with channels that mimic resistance vessels. Anal Chem 76:4849–4855
Ragan DMS, Schmidt EE, MacDonald IC, Groom AC (1988) Spontaneous cyclic contractions of the capillary wall in vivo, impeding red cell flow: a quantitative analysis. Microvasc Res 36:13–30
Sadd MH (2005) Elasticity: theory, applications and numerics. Elsveier, Oxford
Safeukui I, Buffet PA, Deplaine G, Perrot S, Brousse V, Ndour A, Nguyen M, Mercereau-Puijalon O, David PH, Milon G, Mohandas N (2012) Quantitative assessment of sensing and sequestration of spherocytic erythrocytes by the human spleen. Blood 120(2):424–430
Safeukui I, Buffet PA, Deplaine G, Perrot S, Bouusse V et al (2018) Sensing of red blood cells with decreased membrane deformability by the human spleen. Blood Adv 2:2581–2587
Saito H, Yokoi Y, Watanabe S, Tajima J, Kuroda H, Namihisa T (1988) Reticular meshwork of the spleen in rats studied by electron microscopy. Am J Anat 181:235–252
Sato H, Katano M, Takigawa T, Masuda T (2001) Estimation for the elasticity of vascular endothelial cells on the basis of atomic force microscopy and Young’s modulus of gelatin gels. Polym Bull 47:375–381
Schmidt EE, MacDonald IC, Groom AC (1988) Microcirculatory pathways in normal human spleen, demonstrated by scanning electron microscopy of corrosion casts. Am J Anat 181:253–266
Schmidt EE, MacDonald IC, Groom AC (1993) Comparative aspects of splenic microcirculatory pathways in mammals: the region bordering the white pulp. Scanning Microsc 7:613–628
Shah R, Patel T, Freedman JE (2018) Circulating extracellular vesicles in human disease. N Engl J Med 379:958–966
Shen Q, Wu MH, Yuan SY (2009) Endothelial contractile cytoskeleton and microvascular permeability. Cell Health Cytoskelet 1:43–50
Sisquella X, Nebi T, Thompson JK, Whitehead L, Malpede BM et al (2017) Plasmodium falciparum ligand binding to erythrocytes induce alterations in deformability essential for invasion. eLife 6:21083–21094
Song SH, Groom AC (1971) The distribution of red cells in the spleen. Am J Physiol Pharmacol 49:734–743
Song SH, Groom AC (1972) Sequestration and possible maturation of reticulocytes in the normal spleen. Can J Physiol Pharmacol 50:400–406
Song SH, Groom AC (1974) Scanning electron microscope study of the splenic red pulp in relation to the sequestration of immature and abnormal red cells. J Morphol 144:439–452
Sprague RS, Ellsworth ML (2012) Erythrocyte-derived ATP and perfusion distribution: role of intracellular and intercellular communication. Microcirculation 19(5):430–439
Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ (1998) Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol Heart C 275:H1726-171732
Steinger B, Bette M, Schwarzbach H (2011) The open microcirculation in human spleens: a three-dimensional approach. J Histochem Cytochem 59(6):639–648
Thomas CE (1967) An electron- and light-microscope study of sinus structure in perfused rabbit and dog spleens. Am J Anat 120:527–552
Wan J, Ristenpart WD, Stone HA (2008) Dynamics of shear-induced ATP release from red blood cells. PNAS 105:16432–16437
Wautier JL, Paton RC, Wautier MP, Pintigny D, Abadie E, Passa P, Caen JP (1981) N Engl J Med 305:237–242
Wautier JL, Wautier MP (2013) Molecular basis of erythrocyte adhesion to endothelial cells in disease. Clin Hemotol Microcircul 53(1–2):11–21
Wautier JL, Wautier MP, Schmidt AM, Anderson CM, Hori O, Zoukourian C, Capron L, Chappey O, Yan SD, Brett J (1994) Advanced glycation end products (AGEs) on the surface of diabetic erythrocytes bind to the vessel wall via a specific receptor inducing oxidant stress in vasculature: a link between surface-associated AGEs and diabetic complications. Proc Natl Acad Sci USA 91:7742–7746
Wautier MP, Héron E, Picot J, Colin Y, Hermine O, Wautier JL (2011) Red blood phosphatidylserine exposure is responsible for increased erythrocyte adhesion to endothelium in central retinal vein occlusion. J Throm Haemostatis 9(5):1049–1055
Willekens FLA, Weere JM, Groenen-döpp YA, Bregt-Roerdinkholder B, de Pauw B, Bosman GJCGM (2003a) Erythrocyte vesiculation: a self-protective mechanism? Br J Haematol 141:549–556
Willekens FLA, Bregt-Roerdinkholder B, Groenen-döpp YA, Bos HJ, Bosman GJCGM, van den Bos AG, Verkeij AJ, Weere JM (2003b) Haemoglobin loss from erythrocytes in vivo results from spleen-facilitated vesiculation. Blood 101:747–751
Zhu X, Han W, Wang L, Chu H, Zhao J, Xu Y, Wang T, Guo W (2015) Penicillar arterioles of red pulp in residual spleen after subtotal splenectomy due to splenomegaly in cirrhotic patients: a comparative study. Int J Clin Pathol 8(1):711–718
Zhu Q, Salehyar S, Cabrales P, Asaro R (2017) Prospects of human erythrocyte skeleton-bilayer dissociation during splenic flow. Biophys J 113(4):900–912
Zimring JC (2020) Turning over a new leaf on turning over RBCs. Blood 136(14):1569–1570
Acknowledgements
M.D. acknowledges support from NIH R01HL154150.
Author information
Authors and Affiliations
Contributions
R.J. Asaro, M. Dao, and I. MacDonald all contributed to and performed the research and the writing of this paper.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Deformation of a sinus section under the conditions of Model 1 with the endothelial cell (EC) modulus taken as 10 kPa and LEC = 16 μm. The evolution of the longitudinal strain along the ECs (ε LEC = LE33) is shown first under Ppull = 0 - 0.5 mmHg (0 - 66.7 Pa) with no stress fiber contraction, followed by adding 0 to 20% uniform stress fiber contraction in all ECs while holding Ppull = 0.5 mmHg (66.7 Pa) (mp4 2066 KB)
Deformation of a sinus section under the conditions of Model 2 with the endothelial cell (EC) modulus taken as 10 kPa and LEC = 8 μm. The evolution of the longitudinal strain along the ECs (ε LEC = LE33) is shown first under Ppull = 0 - 1.5 mmHg (0 - 200.0 Pa) with no stress fiber contraction, followed by adding 0 to 30% asymmetric stress fiber contraction in two neighboring ECs while holding Ppull = 1.5 mmHg (200.0 Pa) (mp4 1965 KB)
Rights and permissions
About this article
Cite this article
Dao, M., MacDonald, I. & Asaro, R.J. Erythrocyte flow through the interendothelial slits of the splenic venous sinus. Biomech Model Mechanobiol 20, 2227–2245 (2021). https://doi.org/10.1007/s10237-021-01503-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-021-01503-y