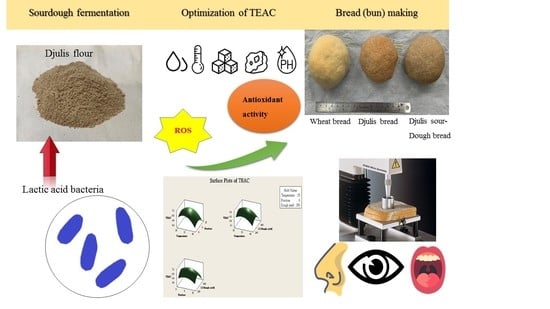
Development and Optimization of Djulis Sourdough Bread Fermented by Lactic Acid Bacteria for Antioxidant Capacity
Abstract
:1. Introduction
2. Results and Discussions
2.1. Strain Selection for Djulis Fermentation
2.2. Optimization of Sourdough Fermentation
2.3. Determination of Antioxidant Capacity
2.4. Analysis of Bread Properties
2.5. Sensory Evaluation of Bread
3. Materials and Methods
3.1. Materials
3.2. Microorganisms and Medium
3.3. Sourdough Bread Production
3.4. Optimization for Djulis Fermentation by the Response Surface Methodology (RSM)
3.5. Determination of Protease Activity and Total Peptide Content
3.6. Determination of Antioxidant Activity
3.7. Determination of Active Compounds
3.8. Property Analysis (Texture Analysis, Bread Specific Volume Analysis, Hole Analysis)
3.9. Nutritional Analysis (Moisture, Ash Content, Crude Fat, Crude Protein, Carbohydrate)
3.10. Sensory Evaluation
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zohary, D.; Harlan, J.R.; Vardi, A. The wild diploid progenitors of wheat and their breeding value. Euphytica 1969, 18, 58–65. [Google Scholar] [CrossRef]
- Erukainure, O.L.; Okafor, J.N.C.; Ogunji, A.; Ukazu, H.; Okafor, E.N.; Eboagwu, I.L. Bambara-wheat composite flour: Rheological behavior of dough and functionality in bread. Food Sci. Nutr. 2016, 4, 852–857. [Google Scholar] [CrossRef] [Green Version]
- Gewehr, M.F.; Pagno, C.H.; Danelli, D.; De Melo, L.M.; Flôres, S.H.; De Jong, E.V. Evaluation of the functionality of bread loaves prepared with quinoa flakes through biological tests. Braz. J. Pharm. Sci. 2016, 52, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Takao, T.; Watanabe, N.; Yuhara, K.; Itoh, S.; Suda, S.; Tsuruoka, Y.; Nakatsugawa, K.; Konishi, Y. Hypocholesterolemic Effect of Protein Isolated from Quinoa (Chenopodium quinoa Willd.) Seeds. Food Sci. Technol. Res. 2005, 11, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Tebben, L.; Shen, Y.; Li, Y. Improvers and functional ingredients in whole wheat bread: A review of their effects on dough properties and bread quality. Trends Food Sci. Technol. 2018, 81, 10–24. [Google Scholar] [CrossRef]
- Rollán, G.C.; Gerez, C.L.; LeBlanc, J.G. Lactic Fermentation as a Strategy to Improve the Nutritional and Functional Values of Pseudocereals. Front. Nutr. 2019, 6, 98. [Google Scholar] [CrossRef] [Green Version]
- Toth, M.; Vatai, G.; Koris, A. Gluten-Free Bread from ingredients and nutrition point of view: A Mini-Review. Emir. J. Food Agric. 2020, 32, 634. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Nutritional constituents of pseudo cereals and their potential use in food systems: A review. Trends Food Sci. Technol. 2018, 75, 170–180. [Google Scholar] [CrossRef]
- Tsai, P.J.; Sheu, C.H.; Wu, P.H.; Sun, Y.F. Thermal and pH stability of betacyanin pigment of Djulis (Chenopodium formosanum) in Taiwan and their relation to antioxidant activity. J. Agric. Food Chem. 2010, 58, 1020–1025. [Google Scholar] [CrossRef]
- Chyau, C.C.; Chu, C.C.; Chen, S.Y.; Duh, P.D. The Inhibitory Effects of Djulis (Chenopodium formosanum) and Its Bioactive Compounds on Adipogenesis in 3T3-L1 Adipocytes. Molecules 2018, 23, 1780. [Google Scholar] [CrossRef] [Green Version]
- Hsu, B.Y.; Lin, S.W.; Inbaraj, B.S.; Chen, B.H. Simultaneous determination of phenolic acids and flavonoids in Chenopodium formosanum Koidz. (djulis) by HPLC-DAD-ESI-MS/MS. J. Pharm. Biomed. Anal. 2017, 132, 109–116. [Google Scholar] [CrossRef]
- Tsai, P.J.; Chen, Y.S.; Sheu, C.H.; Chen, C.Y. Effect of nanogrinding on the pigment and bioactivity of Djulis (Chenopodium formosanum Koidz.). J. Agric. Food Chem. 2011, 59, 1814–1820. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Zheng, J. Lifestyles of sourdough lactobacilli—Do they matter for microbial ecology and bread quality? Int. J. Food Microbiol. 2019, 302, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Stiles, M.E.; Holzapfel, W.H. Lactic acid bacteria of foods and their current taxonomy. Int. J. Food Microbiol. 1997, 36, 1–29. [Google Scholar] [CrossRef]
- Settanni, L.; Moschetti, G. Non-starter lactic acid bacteria used to improve cheese quality and provide health benefits. Food Microbiol. 2010, 27, 691–697. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, X.; Sun, W.; Wang, C.; Li, C.; He, L.; Wang, X.; Tao, H.; Zeng, X. Evaluation and Application of Different Cholesterol-Lowering Lactic Acid Bacteria as Potential Meat Starters. J. Food Prot. 2021, 84, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Albano, C.; Morandi, S.; Silvetti, T.; Casiraghi, M.C.; Manini, F.; Brasca, M. Lactic acid bacteria with cholesterol-lowering properties for dairy applications: In vitro and in situ activity. J. Dairy Sci. 2018, 101, 10807–10818. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Shah, N.P. Concomitant ingestion of lactic acid bacteria and black tea synergistically enhances flavonoid bioavailability and attenuates d-galactose-induced oxidative stress in mice via modulating glutathione antioxidant system. J. Nutr. Biochem. 2016, 38, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Kumar, A.; Kumar, M.; Behare, P.V.; Jain, S.; Yadav, H. Probiotics, their health benefits and applications for developing healthier foods: A review. FEMS Microbiol. Lett. 2012, 334, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Lin, S.-P.; Kuo, T.-C.; Wang, H.-T.; Ting, Y.; Hsieh, C.-W.; Chen, Y.-K.; Hsu, H.-Y.; Cheng, K.-C. Enhanced bioethanol production using atmospheric cold plasma-assisted detoxification of sugarcane bagasse hydrolysate. Bioresour. Technol. 2020, 313, 123704. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.D.; Wu, S.P.; Lin, S.P.; Lum, C.C.; Cheng, K.C. Enhanced active extracellular polysaccharide production from Ganoderma formosanum using computational modeling. J. Food Drug Anal. 2017, 25, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Santoso, S.P.; Chou, C.-C.; Lin, S.-P.; Soetaredjo, F.E.; Ismadji, S.; Hsieh, C.-W.; Cheng, K.C. Enhanced production of bacterial cellulose by Komactobacter intermedius using statistical modeling. Cellulose 2020, 27, 2497–2509. [Google Scholar] [CrossRef]
- Chen, H.Y.; Ting, Y.; Kuo, H.C.; Hsieh, C.W.; Hsu, H.Y.; Wu, C.N.; Cheng, K.C. Enzymatic degradation of ginkgolic acids by laccase immobilized on core/shell Fe(3)O(4)/nylon composite nanoparticles using novel coaxial electrospraying process. Int. J. Biol. Macromol. 2021, 172, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.H.; Hung, W.C.; Lo, K.Y.; Chen, Y.H.; Wan, H.P.; Cheng, K.C. Bioethanol production from taro waste using thermo-tolerant yeast Kluyveromyces marxianus K21. Bioresour. Technol. 2016, 201, 27–32. [Google Scholar] [CrossRef]
- Lai, Y.-T.; Cheng, K.-C.; Lai, C.-N.; Lai, Y.-J. Isolation and identification of aroma producing strain with esterification capacity from yellow water. PLoS ONE 2019, 14, e0211356. [Google Scholar] [CrossRef]
- Filho, A.M.M.; Pirozi, M.R.; Borges, J.T.D.S.; Pinheiro Sant’Ana, H.M.; Chaves, J.B.P.; Coimbra, J.S.D.R. Quinoa: Nutritional, functional, and antinutritional aspects. Crit. Rev. Food Sci. Nutr. 2017, 57, 1618–1630. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Y.; Chen, J.; Sadiq, F.A.; Zhang, G.; Li, Y.; He, G. Prevalence and diversity of lactic acid bacteria in Chinese traditional sourdough revealed by culture dependent and pyrosequencing approaches. LWT-Food Sci. Technol. 2016, 68, 91–97. [Google Scholar] [CrossRef]
- Sakandar, H.; Hussain, R.; Kubow, S.; Sadiq, F.; Huang, W.; Imran, M. Sourdough Bread: A Contemporary Cereal Fermented Product. J. Food Process. Preserv. 2019, 43, e13883. [Google Scholar] [CrossRef]
- Nutter, J.; Fritz, R.; Saiz, A.I.; Iurlina, M.O. Effect of honey supplementation on sourdough: Lactic acid bacterial performance and gluten microstructure. LWT 2017, 77, 119–125. [Google Scholar] [CrossRef]
- Bustos, A.Y.; Gerez, C.L.; Mohtar Mohtar, L.G.; Paz Zanini, V.I.; Nazareno, M.A.; Taranto, M.P.; Iturriaga, L.B. Lactic acid fermentation improved textural behaviour, phenolic compounds and antioxidant activity of chia (Salvia hispanica L.) dough. Food Technol. Biotechnol. 2017, 55, 381–389. [Google Scholar] [CrossRef]
- Coda, R.; Di Cagno, R.; Gobbetti, M.; Rizzello, C.G. Sourdough lactic acid bacteria: Exploration of non-wheat cereal-based fermentation. Food Microbiol. 2014, 37, 51–58. [Google Scholar] [CrossRef]
- Axel, C.; Röcker, B.; Brosnan, B.; Zannini, E.; Furey, A.; Coffey, A.; Arendt, E.K. Application of Lactobacillus amylovorus DSM19280 in gluten-free sourdough bread to improve the microbial shelf life. Food Microbiol. 2015, 47, 36–44. [Google Scholar] [CrossRef]
- Corsetti, A. Technology of sourdough fermentation and sourdough applications. In Handbook on Sourdough Biotechnology; Springer: Berlin, Germany, 2013; pp. 85–103. [Google Scholar]
- Dan-asabe, B.; Galadi, A. Mathematical Modeling of the flexural strength, impact energy and water absorption of a pineapple fibre-HDPE composite using RSM technique. J. Polym. Compos. 2017, 5, 42–49. [Google Scholar]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Banu, I.; Vasilean, I.; Aprodu, I. Effect of lactic fermentation on antioxidant capacity of rye sourdough and bread. Food Sci. Technol. Res. 2010, 16, 571–576. [Google Scholar] [CrossRef]
- Irakli, M.; Mygdalia, A.; Chatzopoulou, P.; Katsantonis, D. Impact of the combination of sourdough fermentation and hop extract addition on baking properties, antioxidant capacity and phenolics bioaccessibility of rice bran-enhanced bread. Food Chem. 2019, 285, 231–239. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Mendes-Ferreira, A.; Benevides, C.M.; Melo, D.; Costa, A.S.; Mendes-Faia, A.; Oliveira, M.B.P. Effect of controlled microbial fermentation on nutritional and functional characteristics of cowpea bean flours. Foods 2019, 8, 530. [Google Scholar] [CrossRef] [Green Version]
- Rizzello, C.G.; Lorusso, A.; Montemurro, M.; Gobbetti, M. Use of sourdough made with quinoa (Chenopodium quinoa) flour and autochthonous selected lactic acid bacteria for enhancing the nutritional, textural and sensory features of white bread. Food Microbiol. 2016, 56, 1–13. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Calasso, M.; Archetti, G.; Rizzello, C.G. Novel insights on the functional/nutritional features of the sourdough fermentation. Int. J. Food Microbiol. 2019, 302, 103–113. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Z.; Shao, J.; Wang, C.; Zhan, C. Effect of fermentation on the peptide content, phenolics and antioxidant activity of defatted wheat germ. Food Biosci. 2017, 20, 141–148. [Google Scholar] [CrossRef]
- Huang, L.; Liu, M.; Huang, H.; Wen, Y.; Zhang, X.; Wei, Y. Recent advances and progress on melanin-like materials and their biomedical applications. Biomacromolecules 2018, 19, 1858–1868. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Lorusso, A.; Russo, V.; Pinto, D.; Marzani, B.; Gobbetti, M. Improving the antioxidant properties of quinoa flour through fermentation with selected autochthonous lactic acid bacteria. Int. J. Food Microbiol. 2017, 241, 252–261. [Google Scholar] [CrossRef]
- Sadeghi, A.; Ebrahimi, M.; Mortazavi, S.A.; Abedfar, A. Application of the selected antifungal LAB isolate as a protective starter culture in pan whole-wheat sourdough bread. Food Control 2019, 95, 298–307. [Google Scholar] [CrossRef]
- Xu, X.; Luo, Z.; Yang, Q.; Xiao, Z.; Lu, X. Effect of quinoa flour on baking performance, antioxidant properties and digestibility of wheat bread. Food Chem. 2019, 294, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Hou, G.G.; Dubat, A. Effects of flour particle size on the quality attributes of reconstituted whole-wheat flour and Chinese southern-type steamed bread. LWT-Food Sci. Technol. 2017, 82, 147–153. [Google Scholar] [CrossRef]
- Rinaldi, M.; Paciulli, M.; Caligiani, A.; Sgarbi, E.; Cirlini, M.; Dall’Asta, C.; Chiavaro, E. Durum and soft wheat flours in sourdough and straight-dough bread-making. J. Food Sci. Technol. 2015, 52, 6254–6265. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhu, F. Formulation and quality attributes of quinoa food products. Food Bioprocess. Technol. 2016, 9, 49–68. [Google Scholar] [CrossRef]
- Guzzo, J.; Desroche, N.; Weidmann, S. Physical and chemical stress factors in lactic acid bacteria. In Biology of Microorganisms on Grapes, in Must and in Wine; Springer: Berlin, Germany, 2017; pp. 397–419. [Google Scholar]
- Karray, F.; Campilho, A.; Cheriet, F. Image Analysis and Recognition, Proceedings of the 14th International Conference, ICIAR 2017, Montreal, QC, Canada, 5–7 July 2017; Springer: Berlin, Germany, 2017; Volume 10317. [Google Scholar]
- Bolívar-Monsalve, J.; Ceballos-González, C.; Ramírez-Toro, C.; Bolívar, G.A. Reduction in saponin content and production of gluten-free cream soup base using quinoa fermented with Lactobacillus plantarum. J. Food Process. Preserv. 2018, 42, e13495. [Google Scholar] [CrossRef]
- Bartkiene, E.; Bartkevics, V.; Krungleviciute, V.; Pugajeva, I.; Zadeike, D.; Juodeikiene, G. Lactic Acid Bacteria Combinations for Wheat Sourdough Preparation and Their Influence on Wheat Bread Quality and Acrylamide Formation. J. Food Sci. 2017, 82, 2371–2378. [Google Scholar] [CrossRef]
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric Assay Using o-Phthaldialdehyde for Determination of Proteolysis in Milk and Isolated Milk Proteins1. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Zou, L.; Fu, C.; Li, P.; Zhao, G. Chemical characterization, antioxidant, immune-regulating and anticancer activities of a novel bioactive polysaccharide from Chenopodium quinoa seeds. Int. J. Biol. Macromol. 2017, 99, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Jeong, S.; Lee, S. Elucidation of rheological properties and baking performance of frozen doughs under different thawing conditions. J. Food Eng. 2020, 284, 110084. [Google Scholar] [CrossRef]
- Kortei, N.K.; Odamtten, G.T.; Obodai, M.; Akonor, P.T.; Wiafe-Kwagyan, M.; Buckman, S.; Mills, S.W.N.O. Sensory evaluation, descriptive textural analysis, and consumer acceptance profile of steamed gamma-irradiated Pleurotus ostreatus (Ex. Fr.) Kummer kept in two different storage packs. Sci. Afr. 2020, 8, e00328. [Google Scholar] [CrossRef]



| Run | Temperature (°C, X1) | Fructose (%, X2) | Dough Yield (X3) | TEAC (mmol/kg, Y) |
|---|---|---|---|---|
| 1 | 35 | 10 | 250 | 6.18 |
| 2 | 40 | 6 | 350 | 6.24 |
| 3 | 35 | 8 | 350 | 6.98 |
| 4 | 35 | 8 | 350 | 6.92 |
| 5 | 35 | 6 | 450 | 6.04 |
| 6 | 30 | 8 | 450 | 6.04 |
| 7 | 30 | 10 | 350 | 6.42 |
| 8 | 35 | 6 | 250 | 6.32 |
| 9 | 35 | 10 | 450 | 5.75 |
| 10 | 30 | 6 | 350 | 6.45 |
| 11 | 35 | 8 | 350 | 6.98 |
| 12 | 40 | 8 | 450 | 5.19 |
| 13 | 40 | 8 | 250 | 5.77 |
| 14 | 40 | 10 | 350 | 5.98 |
| 15 | 30 | 8 | 250 | 6.55 |
| Source | DF | Adj MS | F-Value | p |
|---|---|---|---|---|
| Regression | 9 | 0.41167 | 9.99 | 0.010 ** |
| Linear | 3 | 0.44755 | 10.86 | 0.013 * |
| Temperature | 1 | 0.75537 | 18.33 | 0.008 ** |
| Fructose | 1 | 0.23844 | 5.78 | 0.061 |
| Dough yield | 1 | 0.78291 | 18.99 | 0.007 ** |
| Square | 3 | 0.85519 | 20.75 | 0.003 ** |
| Temperature × Temperature | 1 | 0.85796 | 20.82 | 0.006 ** |
| Fructose × Fructose | 1 | 0.32087 | 7.78 | 0.038 * |
| Dough yield × Dough yield | 1 | 1.71256 | 41.55 | 0.001 *** |
| Interaction | 3 | 0.00655 | 0.16 | 0.919 |
| Temperature × Fructose | 1 | 0.01254 | 0.30 | 0.605 |
| Temperature × Dough yield | 1 | 0.00119 | 0.03 | 0.872 |
| Fructose × Dough yield | 1 | 0.00593 | 0.14 | 0.720 |
| Residual Error | 5 | 0.04122 | ||
| Lack-of-Fit | 3 | 0.04748 | 1.49 | 0.425 > 0.05 |
| Pure Error | 2 | 0.03183 | ||
| Total | 14 |
| Groups | TEACABTS (mmol/kg) | TEACDPPH (mmol/kg) | Total Phenolic Compounds (mg GAE/g) | Total Flavonoid Compounds (mg QE/g) | Total Peptides (mg leucine/g) |
|---|---|---|---|---|---|
| Unfermented grains | 4.12 ± 0.11 a | 4.55 ± 0.21 b | 4.62 ± 0.20 b | 2.14 ± 0.05 b | 1.78 ± 0.11 c |
| Original product | 6.23 ± 0.13 b | 5.04 ± 0.12 a | 6.14 ± 0.38 a | 2.44 ± 0.07 a | 3.20 ± 0.10 b |
| Optimal product | 6.82 ± 0.085 a | 5.63 ± 0.25 a | 6.88 ± 0.1 a | 2.50 ± 0.07 a | 3.81 ± 0.26 a |
| Groups | TEACABTS (mmol/kg) | TEACDPPH (mmol/kg) | Total Phenolic Compounds (mg GAE/g) | Total Flavonoid Compounds (mg QE/g) | Total Peptides (mg leucine/g) |
|---|---|---|---|---|---|
| WB | 1.79 ± 0.30 c | 0.02 ± 0.02 d | 2.01 ± 0.15 c | 0.05 ± 0.002 c | 1.23 ± 0.07 c |
| DB | 3.09 ± 0.03 b | 0.73 ± 0.02 c | 3.59 ± 0.13 b | 0.47 ± 0.01 b | 2.48 ± 0.19 b |
| 20% DSB | 3.55 ± 0.10 a | 0.89 ± 0.07 b | 4.23 ± 0.13 a | 0.55 ± 0.01 a | 3.47 ± 0.05 a |
| 25% DSB | 3.46 ± 0.08 a | 1.12 ± 0.03 a | 4.22 ± 0.22 a | 0.58 ± 0.04 a | 3.35 ± 0.12 a |
| Characteristics | WB | DB | 20% DSB | 25% DSB |
|---|---|---|---|---|
| Specific volume (cm3/g) | 3.91 ± 0.65 a | 3.43 ± 0.62 b | 2.55 ± 0.49 c | 2.67 ± 1.83 c |
| Area (%) | 50.49 ± 0.65 a | 39.42 ± 0.62 c | 44.41 ± 0.49 b | 42.13 ± 1.83 b |
| Particle size (mm2) | 0.20 ± 0.10 b | 0.50 ± 0.00 a | 0.30 ± 0.10 a | 0.40 ± 0.00 a |
| Hardness (g) | 983.10 ± 61.57 c | 973.90 ± 113.10 c | 1789.00 ± 232.70 b | 3121.00 ± 96.81 a |
| Springiness | 1.03 ± 0.05 a | 0.99 ± 0.003 a | 0.9813 ± 0.004 a | 0.9717 ± 0.001 a |
| Cohesiveness | 0.002 ± 0.90 a | 0.001 ± 0.90 a | 0.003 ± 0.88 b | 0.002 ± 0.87 b |
| Chewiness (g) | 919.10 ± 96.46 c | 865.30 ± 99.80 c | 1546.00 ± 189.00 b | 2632.00 ± 87.96 a |
| Resilience | 0.59 ± 0.01 a | 0.56 ± 0.004 b | 0.54 ± 0.009 b,c | 0.51 ± 0.002 d |
| Moisture (%) | 35.16 ± 0.76 | 33.35 ± 0.60 | 32.52 ± 0.10 | 30.33 ± 0.20 |
| Fat (%) | 1.05 ± 0.84 | 1.31 ± 1.1 | 2.11 ± 1.71 | 2.16 ± 1.93 |
| Protein (%) | 8.99 ± 0.11 | 9.89 ± 0.06 | 10.39 ± 0.10 | 10.84 ± 0.15 |
| Carbohydrate (%) | 53.91 ± 0.98 | 54.11 ± 0.53 | 53.98 ± 0.42 | 55.23 ± 0.20 |
| Ash (%) | 1.10 ± 0.06 | 1.55 ± 0.05 | 1.41 ± 0.03 | 1.68 ± 0.09 |
| Groups | Appearance | Flavor | Taste | Texture | Overall Acceptability |
|---|---|---|---|---|---|
| WB | 6.20 ± 0.28 a,b | 5.89 ± 0.28 b | 5.77 ± 0.29 a,b | 5.89 ± 0.27 a,b | 6.00 ± 0.26 a,b |
| DB | 6.06 ± 0.23 b | 5.20 ± 0.21 c | 4.40 ± 0.26 b | 5.03 ± 0.33 c | 4.74 ± 0.27 b |
| 20%DSB | 6.11 ± 0.25 b | 5.74 ± 0.23 b,c | 5.11 ± 0.27 b | 5.71 ± 0.29 a,b,c | 5.31 ± 0.23 b |
| 20%DSBC | 6.89 ± 0.24 a | 6.57 ± 0.21 a | 6.09 ± 0.26 a | 6.46 ± 0.23 a | 6.31 ± 0.23 a |
| Type (%) | WB | DB | 20% DSB | 25% DSB | DSB with 20% Cocoa Powder |
|---|---|---|---|---|---|
| Djulis sourdough | |||||
| djulis | - | - | 20 | 25 | 20 |
| water | 46.6 | 58.25 | 46.6 | ||
| Bread dough | |||||
| Wheat flour | 100 | 80 | 80 | 75 | 80 |
| Djulis flour | - | 20 | - | - | - |
| Water | 60 | 60 | 13.4 | 1.75 | 13.4 |
| Sugar | 5 | 5 | 5 | 5 | 5 |
| Salt | 1 | 1 | 1 | 1 | 1 |
| Yeast | 2 | 2 | 2 | 2 | 2 |
| Oil | 5 | 5 | 5 | 5 | 5 |
| Cocoa powder | 5 | ||||
| Dried cranberry | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-Y.; Hsieh, C.-W.; Chen, P.-C.; Lin, S.-P.; Lin, Y.-F.; Cheng, K.-C. Development and Optimization of Djulis Sourdough Bread Fermented by Lactic Acid Bacteria for Antioxidant Capacity. Molecules 2021, 26, 5658. https://doi.org/10.3390/molecules26185658
Chen H-Y, Hsieh C-W, Chen P-C, Lin S-P, Lin Y-F, Cheng K-C. Development and Optimization of Djulis Sourdough Bread Fermented by Lactic Acid Bacteria for Antioxidant Capacity. Molecules. 2021; 26(18):5658. https://doi.org/10.3390/molecules26185658
Chicago/Turabian StyleChen, Hung-Yueh, Chang-Wei Hsieh, Pin-Cheng Chen, Shin-Pin Lin, Ya-Fen Lin, and Kuan-Chen Cheng. 2021. "Development and Optimization of Djulis Sourdough Bread Fermented by Lactic Acid Bacteria for Antioxidant Capacity" Molecules 26, no. 18: 5658. https://doi.org/10.3390/molecules26185658