Abstract
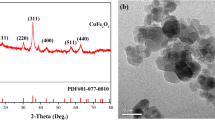
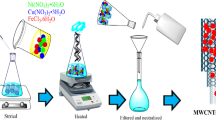
Amlodipine (AML) is an effective drug that has been widely used for hypertension and angina. However, AML is frequently detected in aqueous environments, posing potential risks to human and ecological health. In this study, the degradation of AML via peroxymonosulfate (PMS) activated by CNTs/Co3O4 was investigated. CNTs/Co3O4 was prepared via a facile method, and multiple characterizations suggested that Co3O4 were uniformly dispersed on the surface of MWCNTs-COOH. Experimental results indicated that complete removal of 10 μM AML was achieved within 30 min by using 2 mg/L CNTs/Co3O4 and 4 μM PMS at 25 °C in PBS buffered solution (pH 7.0). The observed pseudo-first-order rate constant was calculated to be 0.1369 min−1. Interestingly, the presence of 100 mM Cl− resulted in a slight enhancement of AML removal rate from 0.0528 to 0.0642 min−1. The addition of 100 mM HCO3−, 5 mg/L Pony Lake fulvic acid (PLFA), or Suwannee River humic acid (SRHA) retarded AML degradation by 15.5, 0.7, and 1.6 times, respectively. As per the quenching experiments, SO4⦁− rather than ⦁OH were verified to be the dominant reactive oxygen species (ROS). Additionally, ten major intermediates were identified using TOF-LC-MS and three associated reaction pathways including ether bond broken, H-abstraction, and hydroxylation were proposed. We outlook these findings to advance the feasibility of organic contaminants removal via CNTs/Co3O4 + PMS systems that have extremely low-level PMS.






Similar content being viewed by others
Data availability
Not applicable.
References
Anipsitakis GP, Dionysiou DD (2004) Transition metal/UV-based advanced oxidation technologies for water decontamination. Appl Catal B Environ 54(3):155–163
Arvaniti OS, Bairamis F, Konstantinou I, Mantzavinos D, Frontistis Z (2020) Degradation of antihypertensive drug valsartan in water matrices by heat and heat/ultrasound activated persulfate: kinetics, synergy effect and transformation products. Chem Eng J Adv 4:100062
Chen CY, Zepp RG (2015) Probing Photosensitization by functionalized carbon nanotubes. Environ Sci Technol 49(23):13835–13843
Chen G, Jirjees F Sr, Al Bawab A, McElnay JC (2018a) Quantification of amlodipine in dried blood spot samples by high performance liquid chromatography tandem mass spectrometry. J Chromatogr B 1072:252–258
Chen X, Oh W-D, Lim T-T (2018b) Graphene- and CNTs-based carbocatalysts in persulfates activation: material design and catalytic mechanisms. Chem Eng J 354:941–976
Dai L, Liu M, Song Y, Liu J, Wang F (2016) Mn3O4-decorated Co3O4 nanoparticles supported on graphene oxide: dual electrocatalyst system for oxygen reduction reaction in alkaline medium. Nano Energy 27:185–195
de Solla SR, Gilroy EA, Klinck JS, King LE, McInnis R, Struger J, Backus SM, Gillis PL (2016) Bioaccumulation of pharmaceuticals and personal care products in the unionid mussel Lasmigona costata in a river receiving wastewater effluent. Chemosphere 146:486–496
Deng J, Xu M, Qiu C, Chen Y, Ma X, Gao N, Li X (2018) Magnetic MnFe2O4 activated peroxymonosulfate processes for degradation of bisphenol A: performance, mechanism and application feasibility. Appl Surf Sci 459:138–147
Du J, Bao J, Liu Y, Ling H, Zheng H, Kim SH, Dionysiou DD (2016a) Efficient activation of peroxymonosulfate by magnetic Mn-MGO for degradation of bisphenol A. J Hazard Mater 320:150–159
Du W, Zhang Q, Shang Y, Wang W, Li Q, Yue Q, Gao B, Xu X (2020) Sulfate saturated biosorbent-derived Co-S@NC nanoarchitecture as an efficient catalyst for peroxymonosulfate activation. Appl Catal B Environ 262:118302
Du Y, Ma W, Liu P, Zou B, Ma J (2016b) Magnetic CoFe2O4 nanoparticles supported on titanate nanotubes (CoFe2O4/TNTs) as a novel heterogeneous catalyst for peroxymonosulfate activation and degradation of organic pollutants. J Hazard Mater 308:58–66
Duan X, Sun H, Shao Z, Wang S (2018) Nonradical reactions in environmental remediation processes: uncertainty and challenges. Appl Catal B Environ 224:973–982
Dong Y, Zhang H, Zhong G, Yao G, Lai B (2021) Cellulose/carbon composites and their applications in water treatment- a review. Chem Eng J 405:126980
Feng Y, Song Q, Lv W, Liu G (2017) Degradation of ketoprofen by sulfate radical-based advanced oxidation processes: kinetics, mechanisms, and effects of natural water matrices. Chemosphere 189:643–651
Ghanbari F, Moradi M (2017) Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: review. Chem Eng J 310:41–62
Guan YH, Ma J, Ren YM, Liu YL, Xiao JY, Lin LQ, Zhang C (2013) Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals. Water Res 47(14):5431–5438
Huang Y, Sheng B, Wang Z, Liu Q, Yuan R, Xiao D, Liu J (2018) Deciphering the degradation/chlorination mechanisms of maleic acid in the Fe(II)/peroxymonosulfate process: an often overlooked effect of chloride. Water Res 145:453–463
Huber S, Remberger M, Kaj L, Schlabach M, Jorundsdottir HO, Vester J, Arnorsson M, Mortensen I, Schwartson R, Dam M (2016) A first screening and risk assessment of pharmaceuticals and additives in personal care products in waste water, sludge, recipient water and sediment from Faroe Islands, Iceland and Greenland. Sci Total Environ 562:13–25
Li C-X, Chen C-B, Lu J-Y, Cui S, Li J, Liu H-Q, Li W-W, Zhang F (2018a) Metal organic framework-derived CoMn2O4 catalyst for heterogeneous activation of peroxymonosulfate and sulfanilamide degradation. Chem Eng J 337:101–109
Li J, Xu M, Yao G, Lai B (2018b) Enhancement of the degradation of atrazine through CoFe2O4 activated peroxymonosulfate (PMS) process: kinetic, degradation intermediates, and toxicity evaluation. Chem Eng J 348:1012–1024
Liang YN, Oh W-D, Li Y, Hu X (2018) Nanocarbons as platforms for developing novel catalytic composites: overview and prospects. Appl Catal A Gen 562:94–105
Liu B, Song W, Wu H, Liu Z, Teng Y, Sun Y, Xu Y, Zheng H (2020) Degradation of norfloxacin with peroxymonosulfate activated by nanoconfinement Co3O4@CNT nanocomposite. Chem Eng J 398:125498
Liu C, Wu B, Chen X (2018a) Sulfate radical-based oxidation for sludge treatment: a review. Chem Eng J 335:865–875
Liu J, Zhou J, Ding Z, Zhao Z, Xu X, Fang Z (2017) Ultrasound irritation enhanced heterogeneous activation of peroxymonosulfate with Fe3O4 for degradation of azo dye. Ultrason Sonochem 34:953–959
Lin K-YA, Chen Y-C, Lin Y-F (2017) LaMO3 perovskites (M=Co, Cu, Fe and Ni) as heterogeneous catalysts for activating peroxymonosulfate in water. Chem Eng Sci 160:96–105
Liu Y, Luo R, Li Y, Qi J, Wang C, Li J, Sun X, Wang L (2018b) Sandwich-like Co3O4/MXene composite with enhanced catalytic performance for Bisphenol A degradation. Chem Eng J 347:731–740
Lu J, Liu F, Chen F, Jin Y, Chen H, Liu D, Cui W (2016) Amlodipine and atorvastatin improve ventricular hypertrophy and diastolic function via inhibiting TNF-alpha, IL-1beta and NF-kappaB inflammatory cytokine networks in elderly spontaneously hypertensive rats. Biomed Pharmacother 83:330–339
Lu S, Wang G, Chen S, Yu H, Ye F, Quan X (2018) Heterogeneous activation of peroxymonosulfate by LaCo1-xCuxO3 perovskites for degradation of organic pollutants. J Hazard Mater 353:401–409
Miao D, Peng J, Zhou X, Qian L, Wang M, Zhai L, Gao S (2018) Oxidative degradation of atenolol by heat-activated persulfate: kinetics, degradation pathways and distribution of transformation intermediates. Chemosphere 207:174–182
Pan X, Yan L, Li C, Qu R, Wang Z (2017) Degradation of UV-filter benzophenone-3 in aqueous solution using persulfate catalyzed by cobalt ferrite. Chem Eng J 326:1197–1209
Pan X, Yan L, Qu R, Wang Z (2018) Degradation of the UV-filter benzophenone-3 in aqueous solution using persulfate activated by heat, metal ions and light. Chemosphere 196:95–104
Peng J, Zhang C, Zhang Y, Shao S, Wang P, Liu G, Dong H, Liu D, Shi J, Cao Z, Liu H, Gao S (2020) Efficient removal of triclosan via peroxymonosulfate activated by a ppb level dosage of Co(II) in water: reaction kinetics, mechanisms and detoxification. Ecotox Environ Safe 198:110676
Peng J, He Y, Zhou C, Su S, Lai B (2021a) The carbon nanotubes-based materials and their applications for organic pollutant removal: a critical review. Chin Chem Lett 32:1626–1636
Peng J, Wang Z, Wang S, Liu J, Zhang Y, Wang B, Gong Z, Wang M, Dong H, Shi J, Liu H, Yan G, Liu G, Gao S, Cao Z (2021b) Enhanced removal of methylparaben mediated by cobalt/carbon nanotubes (Co/CNTs) activated peroxymonosulfate in chloride-containing water: reaction kinetics, mechanisms and pathways. Chem Eng J 409:128176
Pinho MT, Gomes HT, Ribeiro RS, Faria JL, Silva AMT (2015) Carbon nanotubes as catalysts for catalytic wet peroxide oxidation of highly concentrated phenol solutions: towards process intensification. Appl Catal B Environ 165:706–714
Sanganyado E, Lu Z, Fu Q, Schlenk D, Gan J (2017) Chiral pharmaceuticals: a review on their environmental occurrence and fate processes. Water Res 124:527–542
Santos LH, Gros M, Rodriguez-Mozaz S, Delerue-Matos C, Pena A, Barcelo D, Montenegro MC (2013) Contribution of hospital effluents to the load of pharmaceuticals in urban wastewaters: identification of ecologically relevant pharmaceuticals. Sci Total Environ 461-462:302–316
Shao S, Qian L, Zhan X, Wang M, Lu K, Peng J, Miao D, Gao S (2020) Transformation and toxicity evolution of amlodipine mediated by cobalt ferrite activated peroxymonosulfate: effect of oxidant concentration. Chem Eng J 382:123005
Sharma J, Mishra IM, Dionysiou DD, Kumar V (2015) Oxidative removal of Bisphenol A by UV-C/peroxymonosulfate (PMS): kinetics, influence of co-existing chemicals and degradation pathway. Chem Eng J 276:193–204
Shi P, Dai X, Zheng H, Li D, Yao W, Hu C (2014) Synergistic catalysis of Co3O4 and graphene oxide on Co3O4/GO catalysts for degradation of Orange II in water by advanced oxidation technology based on sulfate radicals. Chem Eng J 240:264–270
Song Q, Feng Y, Wang Z, Liu G, Lv W (2019) Degradation of triphenyl phosphate (TPhP) by CoFe2O4-activated peroxymonosulfate oxidation process: kinetics, pathways, and mechanisms. Sci Total Environ 681:331–338
Tan C, Gao N, Fu D, Deng J, Deng L (2017) Efficient degradation of paracetamol with nanoscaled magnetic CoFe2O4 and MnFe2O4 as a heterogeneous catalyst of peroxymonosulfate. Sep Purif Technol 175:47–57
Tian W, Zhang H, Qian Z, Ouyang T, Sun H, Qin J, Tadé MO, Wang S (2018) Bread-making synthesis of hierarchically Co@C nanoarchitecture in heteroatom doped porous carbons for oxidative degradation of emerging contaminants. Appl Catal B Environ 225:76–83
Tiwari RN, Shah N, Bhalani V, Mahajan A (2015) LC, MS (n) and LC-MS/MS studies for the characterization of degradation products of amlodipine. J Pharm Anal 5(1):33–42
Toma L, Sanda GM, Raileanu M, Stancu CS, Niculescu LS, Sima AV (2020) Ninjurin-1 upregulated by TNFalpha receptor 1 stimulates monocyte adhesion to human TNFalpha-activated endothelial cells; benefic effects of amlodipine. Life Sci 249:117518
Wacławek S, Lutze HV, Grübel K, Padil VVT, Černík M, Dionysiou DD (2017) Chemistry of persulfates in water and wastewater treatment: a review. Chem Eng J 330:44–62
Wang J, Wang S (2018) Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem Eng J 334:1502–1517
Wang M, Shi H, Shao S, Lu K, Wang H, Yang Y, Gong Z, Zuo Y, Gao S (2022) Montmorillonite promoted photodegradation of amlodipine in natural water via formation of surface complexes. Chemosphere 286:131641
Yao J, Zeng X, Wang Z (2017) Enhanced degradation performance of sulfisoxazole using peroxymonosulfate activated by copper-cobalt oxides in aqueous solution: kinetic study and products identification. Chem Eng J 330:345–354
Ye T, Wei Z, Spinney R, Tang CJ, Luo S, Xiao R, Dionysiou DD (2017) Chemical structure-based predictive model for the oxidation of trace organic contaminants by sulfate radical. Water Res 116:106–115
Zeng L, Liu Q, Van der Bruggen B, Tang K, Yi X, Wang G (2020) An integrated separation process for recovery and enantioseparation of amlodipine from wastewater: supported liquid membrane-aqueous/organic phase crystallization. Sep Purif Technol 248:117121
Zhang L, Song P, Long H, Meng M, Yin Y, Xi R (2017) Magnetism based electrochemical immunosensor for chiral separation of amlodipine. Sensors Actuators B Chem 248:682–689
Zhao H, Ji Y, Kong D, Lu J, Yin X, Zhou Q (2019) Degradation of iohexol by Co2+ activated peroxymonosulfate oxidation: kinetics, reaction pathways, and formation of iodinated byproducts. Chem Eng J 373:1348–1356
Zhou H, Peng J, Li J, You J, Lai L, Liu R, Ao Z, Yao G, Lai B (2021) Metal-free black-red phosphorus as an efficient heterogeneous reductant to boost Fe3+/Fe2+ cycle for peroxymonosulfate activation. Water Res 188:116529
Zhou Y, Xiang Y, He Y, Yang Y, Zhang J, Luo L, Peng H, Dai C, Zhu F, Tang L (2018) Applications and factors influencing of the persulfate-based advanced oxidation processes for the remediation of groundwater and soil contaminated with organic compounds. J Hazard Mater 359:396–407
Zhu J, Wang J, Shan C, Zhang J, Lv L, Pan B (2019) Durable activation of peroxymonosulfate mediated by Co-doped mesoporous FePO4 via charge redistribution for atrazine degradation. Chem Eng J 375:122009
Acknowledgements
We wish to thank anonymous reviewers for their comments, which improved the article greatly.
Funding
This work was supported by the Project funded by China Postdoctoral Science Foundation (2018M632783 and 2018M642758), the Scientific Research Foundation of Henan Normal University (5101219470210, 2021PL26), and Henan provincial key science and technology research project (212102210475).
Author information
Authors and Affiliations
Contributions
J.P. contributed to the conception and design of the experiment. Y.C., Z.W., J.L., and S.W. carried out the experiments and wrote the manuscript text. Y.Z. contributed to the writing of the manuscript. S.S. performed the TOF-LC-MS analysis. D.L., Y.Z., J.S., H.L., G.Y., Z.C., and S.G. contributed to the writing of the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Yes.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Ricardo A. Torres-Palma
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOC 948 kb)
Rights and permissions
About this article
Cite this article
Peng, J., Chang, Y., Wang, Z. et al. Amlodipine removal via peroxymonosulfate activated by carbon nanotubes/cobalt oxide (CNTs/Co3O4) in water. Environ Sci Pollut Res 29, 11091–11100 (2022). https://doi.org/10.1007/s11356-021-16399-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16399-5