Abstract—
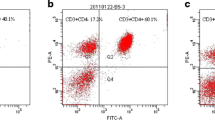
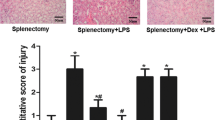
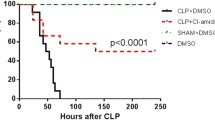
Severe hemorrhagic shock leads to excessive inflammation and immune dysfunction, which results in high mortality related to mesenteric lymph return. A recent study showed that stellate ganglion block (SGB) increased the survival rate in rats suffering hemorrhagic shock. However, whether SGB ameliorates immune dysfunction induced by hemorrhagic shock remains unknown. The aim of the present study was to verify the favorable effects of SGB on the proliferation and function of splenic CD4 + T cells isolated from rats that underwent hemorrhagic shock and to investigate the mechanism related to the SGB interaction with autophagy and posthemorrhagic shock mesenteric lymph (PHSML). Male rats underwent SGB or sham SGB and conscious acute hemorrhage followed by resuscitation and multiple treatments. After 3 h of resuscitation, splenic CD4 + T cells were isolated to measure proliferation and cytokine production following stimulation with ConA in vitro. CD4 + T cells isolated from normal rats were treated with PHSML drained from SBG-treated rats, and proliferation, cytokine production, and autophagy biomarkers were detected. Hemorrhagic shock reduced CD4 + T cell proliferation and production of interleukin (IL)-2, IL-4, and tumor necrosis factor-α–induced protein 8–like 2 (TIPE2). SGB or administration of the autophagy inhibitor 3-methyladenine (3-MA) normalized these indicators. In contrast, administration of rapamycin (RAPA) autophagy agonist or intravenous injection of PHSML inhibited the beneficial effects of SGB on CD4 + T cells from hemorrhagic shocked rats. Furthermore, PHSML incubation decreased proliferation and cytokine production, increased LC3 II/I and Beclin-1 expression, and reduced p-PI3K and p-Akt expression in normal CD4 + T cells. These adverse effects of PHSML were also abolished by 3-MA administration, as well as incubation with PHSML obtained from SGB-treated rats. SGB improves splenic CD4 + T cell function following hemorrhagic shock, which is related to the inhibition of PHSML-mediated autophagy.




Similar content being viewed by others
Availability of Data and Materials
The data generated for this study are available on request to the corresponding author.
References
Cannon, J.W. 2018. Hemorrhagic Shock. New England Journal of Medicine 378 (4): 370–379.
Brochner, A.C., and P. Toft. 2009. Pathophysiology of the systemic inflammatory response after major accidental trauma. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine 17: 43.
Menger, M.D., and B. Vollmar. 2004. Surgical trauma: Hyperinflammation versus immunosuppression?. Langenbecks Archives of Surgery 389 (6): 475–484.
Moore, F.A., B.A. McKinley, and E.E. Moore. 2004. The next generation in shock resuscitation. Lancet 363 (9425): 1988–1996.
Yao, F., Y.Q. Lu, J.K. Jiang, et al. 2017. Immune recovery after fluid resuscitation in rats with severe hemorrhagic shock. Journal of Zhejiang University Science B 18 (5): 402–409.
de St Groth, B.F. 2012. Regulatory T-cell abnormalities and the global epidemic of immuno-inflammatory disease. Immunology and Cell Biology 90 (3): 256–259.
Katlama, C., S.G. Deeks, B. Autran, et al. 2013. Barriers to a cure for HIV: New ways to target and eradicate HIV-1 reservoirs. Lancet 381 (9883): 2109–2117.
Liu, H., Z.G. Zhao, L.Q. Xing, et al. 2015. Post-shock mesenteric lymph drainage ameliorates cellular immune function in rats following hemorrhagic shock. Inflammation 38 (2): 584–594.
Tiesi, G., D. Reino, L. Mason, et al. 2013. Early trauma-hemorrhage-induced splenic and thymic apoptosis is gut-mediated and toll-like receptor 4-dependent. Shock 39 (6): 507–513.
Deitch, E.A. 2010. Gut lymph and lymphatics: A source of factors leading to organ injury and dysfunction. Annals of New York Academy of Sciences 1207 (Suppl 1): E103-111.
Deitch, E.A. 2012. Gut-origin sepsis: Evolution of a concept. Surgeon 10 (6): 350–356.
Yang, X., Z. Shi, X. Li, et al. 2015. Impacts of stellate ganglion block on plasma NF-kappaB and inflammatory factors of TBI patients. International Journal of Clinical and Experimental Medicine 8 (9): 15630–15638.
Zhang, J., X.R. Lin, Y.P. Zhang, et al. 2019. Blockade of stellate ganglion remediates hemorrhagic shock-induced intestinal barrier dysfunction. Journal of Surgical Research 244: 69–76.
Jacquin, E., and L. Apetoh. 2018. Cell-intrinsic roles for autophagy in modulating CD4 T cell functions. Frontiers in Immunology 9: 1023.
Sun, G.X., Y.X. Guo, Y.P. Zhang, et al. 2016. Posthemorrhagic shock mesenteric lymph enhances monolayer permeability via F-actin and VE-cadherin. Journal of Surgical Research 203 (1): 47–55.
Jiang, L.N., Y.L. Mi, L.M. Zhang, et al. 2020. Engagement of posthemorrhagic shock mesenteric lymph on CD4(+) T Lymphocytes in vivo and in vitro. Journal of Surgical Research 256: 220–230.
Wang, D., Y. Ma, Z. Li, et al. 2012. The role of AKT1 and autophagy in the protective effect of hydrogen sulphide against hepatic ischemia/reperfusion injury in mice. Autophagy 8 (6): 954–962.
DiJoseph, J.F., R.N. Sharma, and J.Y. Chang. 1992. The effect of rapamycin on kidney function in the Sprague-Dawley rat. Transplantation 53 (3): 507–513.
Huang, R., M. Shen, Y. Yu, et al. 2020. Physicochemical characterization and immunomodulatory activity of sulfated Chinese yam polysaccharide. International Journal of Biological Macromolecules 165 (Pt A): 635–644.
Cui, W.X., M. Yang, H. Li, et al. 2020. Polycyclic furanobutenolide-derived norditerpenoids from the South China Sea soft corals Sinularia scabra and Sinularia polydactyla with immunosuppressive activity. Bioorganic Chemistry 94: 103350.
Cronkite, D.A., and T.M. Strutt. 2018. The regulation of inflammation by innate and adaptive lymphocytes. Journal of Immunology Research 2018: 1467538.
Angele, M.K., and I.H. Chaudry. 2005. Surgical trauma and immunosuppression: Pathophysiology and potential immunomodulatory approaches. Langenbecks Archives of Surgery 390 (4): 333–341.
Bagley, J., T. Sawada, Y. Wu, et al. 2000. A critical role for interleukin 4 in activating alloreactive CD4 T cells. Nature Immunology 1 (3): 257–261.
Sun, H., S. Gong, R.J. Carmody, et al. 2008. TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell 133 (3): 415–426.
Bellavance, M.A., and S. Rivest. 2014. The HPA - Immune axis and the immunomodulatory actions of glucocorticoids in the brain. Frontiers in Immunology 5: 136.
Vasanthakumar, A., D. Chisanga, J. Blume, et al. 2020. Sex-specific adipose tissue imprinting of regulatory T cells. Nature.
Kipnis, J. 2016. Multifaceted interactions between adaptive immunity and the central nervous system. Science 353 (6301): 766–771.
Mravec, B. (2019). Chemical sympathectomy attenuates lipopolysaccharide-induced increase of plasma cytokine levels in rats pretreated by ACTH. Journal of Neuroimmunology 337: 577086.
Moriyama, S., J.R. Brestoff, A.L. Flamar, et al. 2018. beta2-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science 359 (6379): 1056–1061.
Vujnovic, I., I. Pilipovic, N. Jasnic, et al. 2019. Noradrenaline through beta-adrenoceptor contributes to sexual dimorphism in primary CD4+ T-cell response in DA rat EAE model?. Cellular Immunology 336: 48–57.
Qiao, G., M.J. Bucsek, N.M. Winder, et al. 2019. beta-Adrenergic signaling blocks murine CD8(+) T-cell metabolic reprogramming during activation: A mechanism for immunosuppression by adrenergic stress. Cancer Immunology Immunotherapy 68 (1): 11–22.
Horvathova, L., A. Tillinger, I. Sivakova, et al. 2015. Chemical sympathectomy increases neutrophil-to-lymphocyte ratio in tumor-bearing rats but does not influence cancer progression. Journal of Neuroimmunology 278: 255–261.
Mani, SK. (2018). Neuroendocrine regulation of reproduction, stress, inflammation and energy homeostasis. Journal of Neuroendocrinology 30(10): e12648.
Smith, S.M., and W.W. Vale. 2006. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues in Clinical Neuroscience 8 (4): 383–395.
Yokoyama, M., H. Nakatsuka, Y. Itano, et al. 2000. Stellate ganglion block modifies the distribution of lymphocyte subsets and natural-killer cell activity. Anesthesiology 92 (1): 109–115.
Yin, X., H. Xin, S. Mao, et al. 2019. The role of autophagy in sepsis: Protection and injury to organs. Frontiers in Physiology 10: 1071.
Tao, S., and I. Drexler. 2020. Targeting autophagy in innate immune cells: Angel or demon during infection and vaccination?. Frontiers in Immunology 11: 460.
Zhang, L., J.S. Cardinal, P. Pan, et al. 2012. Splenocyte apoptosis and autophagy is mediated by interferon regulatory factor 1 during murine endotoxemia. Shock 37 (5): 511–517.
Rao, Z., X. Pan, H. Zhang, et al. 2017. Isoflurane preconditioning alleviated murine liver ischemia and reperfusion injury by restoring AMPK/mTOR-mediated autophagy. Anesthesia and Analgesia 125 (4): 1355–1363.
Yang, Z., and D.J. Klionsky. 2010. Mammalian autophagy: Core molecular machinery and signaling regulation. Current Opinion in Cell Biology 22 (2): 124–131.
Mizushima, N., and M. Komatsu. 2011. Autophagy: Renovation of cells and tissues. Cell 147 (4): 728–741.
Ravikumar, B., S. Sarkar, J.E. Davies, et al. 2010. Regulation of mammalian autophagy in physiology and pathophysiology. Physiological Reviews 90 (4): 1383–1435.
Chen, Y., and D.J. Klionsky. 2011. The regulation of autophagy - unanswered questions. Journal of Cell Science 124 (Pt 2): 161–170.
Saha, S., D.P. Panigrahi, S. Patil, et al. 2018. Autophagy in health and disease: A comprehensive review. Biomedicine and Pharmacotherapy 104: 485–495.
Chung, Y., J. Lee, S. Jung, et al. 2018. Dysregulated autophagy contributes to caspase-dependent neuronal apoptosis. Cell Death and Disease 9 (12): 1189.
Wang, S., X. Xu, Y. Hu, et al. 2019. Sotetsuflavone induces autophagy in non-small cell lung cancer through blocking PI3K/Akt/mTOR signaling pathway in vivo and in vitro. Frontiers in Pharmacology 10: 1460.
Maejima, Y., M. Isobe, and J. Sadoshima. 2016. Regulation of autophagy by Beclin 1 in the heart. Journal of Molecular and Cellular Cardiology 95: 19–25.
Fan, X.J., Y. Wang, L. Wang, et al. 2016. Salidroside induces apoptosis and autophagy in human colorectal cancer cells through inhibition of PI3K/Akt/mTOR pathway. Oncology Reports 36 (6): 3559–3567.
Wang, Z., L. Zhou, X. Zheng, et al. 2017. Autophagy protects against PI3K/Akt/mTOR-mediated apoptosis of spinal cord neurons after mechanical injury. Neuroscience Letters 656: 158–164.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81770492).
Author information
Authors and Affiliations
Contributions
Y.L., H.D., L.J., C.W., M.Y., L.Z., H.Z., Z.Z., and Z.L. performed majority of the animal experiment and laboratory work; Y.L. acquired and analyzed the data; C.N. and Z.Z. were involved in the conception and design of the study and data interpretation and critically revised the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
All the protocols were approved by the Animal Care Committee of Hebei North University (Zhangjiakou, China).
Consent for Publication
All the authors agree to publish this manuscript.
Conflict of Interest
No benefits in any form have been received or will be received from a commercial association related directly or indirectly to the subject of this article. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Y., Du, HB., Jiang, LN. et al. Stellate Ganglion Block Improves the Proliferation and Function of Splenic CD4 + T Cells Through Inhibition of Posthemorrhagic Shock Mesenteric Lymph–Mediated Autophagy. Inflammation 44, 2543–2553 (2021). https://doi.org/10.1007/s10753-021-01523-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01523-x