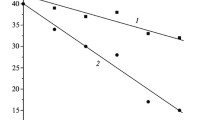
The interaction of lead silicate glasses Ñ87-2 and Ñ78-4 with dilute solutions of hydrofluoric acid was investigated. It is shown that an increase in the temperature and concentration of the HF solution does not lead to an increase in the reaction rate. The obtained results are explained by the fact that, along with the formation of soluble salts, complex compounds with limited solubility appear and on precipitating onto a surface prevent the glass components from entering the solution.




Similar content being viewed by others
References
O. S. Molchanova, L. A. Orlova, and S. E. Krasikov, “On the interaction of porous glass with alkali and hydrofluoric acid,” Fiz. Khim. Stekla, 4(2), 451 – 458 (1989).
S. K. Kulov, Surface Quality of MCP. Part 3. Chemical Properties of the Surface of Silicate Glasses [in Russian], SKGTU, Vladikavkaz (2002).
A. A. Belyustin, Yu. V. Roshchina, and I. S. Ivanovskaya, “Composition of surface layers formed upon interaction with solutions and electrode properties of alkali aluminum borosilicate glasses,” Glass Phys. Chem., 22(1), 54 – 62 (1996).
N. V. Kalinina, Interaction of Water, Acidic Solutions, and Alkaline Solutions with Lead-Silicate and Borate-Barium Glasses, Author’s Abstract of Candidate’s Thesis [in Russian], KBSU, Nalchik (2007).
V. A. Tolmachev, M. A. Okatov, et al., “Interaction of lead-silicate glass with dilute solutions of hydrofluoric acid,” Fiz. Khim. Stekla, 16(1), 11 – 13 (1990).
A. Kh. Kerefov, O. G. Ashkhotov, and N. V. Kalinina, “Method of studying the kinetics of interaction of glasses with aqueous solutions,” Prikl. Fiz., No. 4, 44 – 46 (2005).
I. B. Ashkhotova, Influence of Physical and Chemical Operations on the Process of Forming the Final-Control Surface of Microchannel Plates, Author’s Abstract of Candidate’s Thesis [in Russian], SKSTU, Vladikavkaz (2003).
I. B. Ashkhotova and O. G. Ashkhotov, “Chemical stability of non-etched blanks of microchannel plates in dilute hydrofluoric acid solutions,” in: Abstracts of Reports at the Radional Conf. on Vacuum Electronics in the North Caucasus, Nalchik, 24 – 29 Sept. 2001 [in Russian], Nalchik (2001), pp. 43–44.
S. K. Kulov and E. N. Makarov, “Principles of a new version of the basic technology of MCP,” in: Abstracts of Reports at the International Sci.-Tech. Conf., 13 – 19 July 2008 [in Russian], Nalchik (2008), pp. 55 – 56.
The work was performed in part using the equipment of the Laboratory of Surface Physics and Catalysis as part of the state assignment for SOGU.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Steklo i Keramika, No. 5, pp. 40 – 43, May, 2021.
Rights and permissions
About this article
Cite this article
Ashkhotov, O.G., Magkoev, T.T. & Ashkhotova, I.B. Interaction of Lead-Silicate Glasses with Dilute Hydrofluoric Acid Solutions. Glass Ceram 78, 204–206 (2021). https://doi.org/10.1007/s10717-021-00379-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10717-021-00379-9