Abstract
Antifibrotic therapy (AFT) slows disease progression in patients with idiopathic pulmonary fibrosis (IPF). The Gender-Age-Physiology (GAP) index, was developed based on data at IPF diagnosis before the introduction of AFT and has not been evaluated in the AFT context. Further, recent advances have revealed the importance of body-composition factors in prognosis of IPF treated with AFT. This multi-centre, retrospective study aimed to evaluate the GAP index and body mass index (BMI) at the time of AFT initiation for predicting prognosis in patients with IPF. This study included two patient cohorts of IPF receiving AFT, Hamamatsu cohort (n = 110) and Seirei cohort (n = 119). The distribution of GAP stages I, II, and III was 38.2%, 43.6%, and 18.2%, respectively, in Hamamatsu cohort; in Seirei cohort, it was 41.2%, 50.4%, and 8.4%, respectively. In both cohorts, the GAP index distinctly classified prognosis into three groups (log-rank test). Interestingly, a lower BMI showed prognostic value independent of the GAP index in multivariate analyses. Subsequently, combining the GAP index with BMI at AFT initiation successfully divided the patients with IPF into four distinct prognoses. Assessment of the GAP index and BMI measurement at AFT initiation are important for predicting prognosis in patients with IPF.
Similar content being viewed by others
Introduction
Idiopathic pulmonary fibrosis (IPF) is a fibrotic progressive interstitial lung disease (ILD) characterised by declined pulmonary function and overall poor prognosis1,2. The Gender-Age-Physiology (GAP) index, a simple point-scoring calculator of multidimensional prognostic staging system, was originally proposed and validated in 2012 for the prediction of 1, 2 and 3-year mortalities in IPF3. The GAP index calculates the baseline IPF characteristics and has shown excellent prognostic-group separation ability.
After development of the GAP index, antifibrotic therapy (AFT), i.e. pirfenidone and nintedanib, was established and recommended for IPF treatment in the international guideline of 20152. The pirfenidone and nintedanib slow disease progression by reducing the annual decline of forced vital capacity (FVC) in patients with IPF4,5,6,7. Further, AFT was reported to reduce the decline of FVC in patients with other ILD types, including systemic sclerosis-associated ILD8 and progressive fibrosing ILDs9,10. Based on its documented ability to delay lung-function deterioration, AFT is considered to reduce the risk of mortality11,12. However, the utility of the GAP index in the context of AFT has not been fully evaluated, and the development of a simple and easily applicable prognostic staging system for AFT is expected.
A lower body mass index (BMI) was previously reported to be associated with poor outcome in patients with IPF. Recently, the clinical implication of sarcopenia, which is characterised by progressive and generalised skeletal disorder involving accelerated loss of muscle mass and function, has been highlighted in various diseases14,15. These metabolic dysfunctions, partly represented as muscle wasting and body-weight loss, are frequently found in patients with various respiratory-disease types, including ILD16,17,18,19. Importantly, muscle wasting and body-weight loss have been associated with poor outcome in patients with IPF13,18,19,20,21. These previous studies suggested that preventing skeletal-muscle wasting as well as preserving body weight and lung function are important for the management of patients with IPF.
This multi-centre, retrospective, two-cohort study aimed to evaluate the GAP index at AFT initiation in patients with IPF. Additionally, this study also assessed the value of the BMI and of combined BMI and GAP index assessment for prognosis discrimination in patients with IPF treated with AFT.
Results
Clinical characteristics
The clinical characteristics of the patients with IPF at the time of AFT initiation are shown in Table 1 and Fig. 1. Most patients were approximately 70 years old and 80% of the patients were male in both cohorts. Pirfenidone was commonly used in the Hamamatsu cohort, and surgical lung biopsy was frequently performed in the Hamamatsu cohort. The median follow-up period since AFT was initiated was approximately 2 years, with 70 patients having more than a 3-year observation period and 43 patients having more than a 5-year observation period. The number of patients with previous history of AE at AFT initiation tended to be higher in the Seirei cohort: 16 (13.4%) vs. 7 (6.4%), respectively. The pulmonary function test showed severe-to-moderate impairment of spirometry and decreased %DLCO in both cohorts. The levels of serum albumin were slightly lower in the patients in the Seirei cohort. Long-term oxygen therapy (LTOT) and immunosuppressants were more frequently prescribed in the Seirei cohort. Immunosuppressants were mainly initiated due to acute exacerbation (AE), and some were prescribed before the PANTHOR trial22.
Assessment of the GAP stage in patients with IPF at the time of AFT initiation
The distributions of patients with IPF at the time of AFT initiation according to the GAP index are shown in Table 2. The frequencies of GAP stages I and stage II were approximately 40%. Among them, GAP stage II was most frequent in both cohorts. Meanwhile, the proportion of GAP stage III was lower than 20% in both cohorts, and the proportion of GAP stage III was lower in the Seirei cohort than in the Hamamatsu cohort; especially, that in the Seirei cohort was 8.4%.
Prognostic classification of the GAP index in patients with IPF treated with AFT
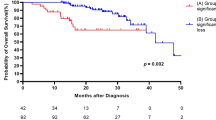
During the follow-up period, 122 deaths were noted. There were no significant differences in causes of death between the two cohorts (Table 3). In both cohorts, approximately 60% of patients died because of chronic respiratory failure, whereas the incidence of lung cancer was lower than 10%. The survival analyses according to the GAP index are shown in Fig. 2 and Supplementary-Table S1. In both cohorts, the GAP index successfully divided the prognosis of patients with IPF treated with AFT into three groups with distinct prognosis. The discrimination performance of the GAP index in patients of the combined cohorts was 0.675 (C-statistics). Among the components of the GAP index, FVC (%) and DLCO (%) also yielded prognostic separation in the combined-cohort patients (Fig. 3), but not the ‘Age’ and ‘Gender’ factors (not shown). The C-index of the ‘GAP index: FVC (%)’ and ‘GAP index: DLCO (%)’ were 0.668 and 0.655, respectively.
Prognostic classifications according to the GAP index at initiation of antifibrotic therapy in patients with IPF. Kaplan–Meier curves based on the data of the patients with IPF of the Hamamatsu cohort (A), Seirei cohort (B), and combined cohort (C) according to the GAP stage. P values were determined using the log-rank test. GAP Gender-Age-Physiology, IPF idiopathic pulmonary fibrosis.
Prognostic classifications according to GAP index: FVC (%) and GAP index: DLCO (%). Kaplan–Meier curves based on the data of the patients with IPF of the Hamamatsu cohort (A,D), Seirei cohort (B,E), and combined cohort (C,F) according to GAP stage: FVC (%) and GAP index: DLCO (%). P values were determined using the log-rank test. GAP Gender-Age-Physiology, FVC forced vital capacity, DLCO diffuse capacity of the lung for carbon monoxide, IPF idiopathic pulmonary fibrosis.
Prognostic factors in patients with IPF treated with AFT
The univariate and multivariate analyses are presented in Table 4. As shown, the GAP index and its components, %FVC and %DLCO, were found to be significant prognostic factors in the multivariate analyses. Additionally, a lower BMI was a significant prognostic factor independent of the GAP index in the multivariate analyses.
Prognostic value of the BMI at AFT initiation in patients with IPF
As the BMI showed prognostic value independent of the GAP index, we performed combined assessment of the GAP index with the BMI, i.e., GAP plus BMI, for IPF prognosis. Interestingly, a reverse J-shaped association was seen between the BMI and mortality rate in the Cox proportional hazards regression model (Supplementary-Figure S1). According to the ROC analyses, we identified a BMI 24 kg/m2 as the optimal cut-off. If the value of the BMI was lower than the cut-off value, the severity of the GAP stage was advanced by one stage. For example, the patients with GAP stage I who had BMI < 24 kg/m2 were categorized as GAP plus BMI stage II. Subsequently GAP plus BMI classified patients into four groups. The survival analyses according to GAP plus BMI are shown in Fig. 4. For clinical relevance, survival curves applying BMI < 20 kg/m2 (C-statistics 0.688) were also shown in Supplementary-Figure S2. GAP plus BMI was successful in distinguishing the prognoses in the combined-cohort patients with IPF (C-statistics 0.698). To calculate model improvement by GAP plus BMI at the 1-year, 3-year, and 5-year survivals, we applied IDI and NRI analyses. GAP plus BMI improved the discriminative performance of 3-year survival at 21% compared to that provided by the GAP index alone, although statistical significance was not reached (Table 5).
Prognostic classifications according to GAP plus BMI. Kaplan–Meier curves based on the data of the patients with IPF of the Hamamatsu cohort (A), Seirei cohort (B), and combined cohort (C) according to GAP plus BMI. P values were determined using the log-rank test. GAP Gender-Age-Physiology, BMI body mass index, IPF idiopathic pulmonary fibrosis.
Discussion
The present study retrospectively examined the utility of the GAP index and of the BMI for prognosis prediction in patients with IPF treated with AFT. First, the GAP index at AFT initiation yielded clear prognostic distinction in the two cohorts of patients with IPF. Next, as BMI showed prognostic value independent of the GAP index, we evaluated the prognosis using the GAP index in conjunction with BMI: GAP plus BMI. Assessment of GAP plus BMI at AFT initiation successfully divided the patients into four groups with distinct prognoses. Our data suggested the clinical usefulness of the GAP index and importance of BMI assessment at AFT initiation in patients with IPF.
The GAP index was developed as a clinical baseline prediction model to distinguish prognostic groups in IPF based on data obtained at the time of diagnosis3. The GAP index is an easy and simple method that enables the assessment of cross-sectional data, without need for longitudinal data, which is advantageous. Accordingly, the GAP index is widely used and its utility has been shown in other types of ILD including unclassifiable ILD, chronic hypersensitive pneumonitis, and connective tissue disease-associated ILD23,24. However, at the time of GAP index development, no pharmacological therapy including pirfenidone was recommended in the international guideline25. Thus, the clinical implications of the GAP index in AFT have not been fully evaluated. In this regard, this study examined the prognostic classifications of the GAP index with two cohorts of patients with IPF treated with AFT and showed that assessment of the GAP index at AFT initiation clearly distinguished the prognoses.
The most common cause of mortality in these cohorts was chronic respiratory failure (57.4%) followed by AE (23.8%), whereas the lung-cancer incidence was very low at 5.7%. Interestingly, an epidemiological study conducted in Japan between 2003 and 2007 (i.e. before the introduction of AFT) reported the incidence of AE, chronic respiratory failure, and lung cancer at 40%, 24%, and 11%, respectively26; the lung-cancer incidence was twice as high as that in our cohort and resulted in an increase in the combined incidence of chronic respiratory failure and AE after AFT development. Although AE can occur in patients with preserved lung function, low FVC was reported as the most consistent risk factor for AE in IPF, and low DLCO was also identified as a well-known risk factor for AE in an international statement27. Therefore, these changes in cause of death may have positively contributed to mortality prediction using the GAP index at AFT initiation.
Given that the GAP index is a baseline risk-prediction model largely based on physiological factors, these issues also imply their own limitations. Disease behaviour or exercise capacity are not required to calculate the GAP stage. Indeed, both baseline and changes in spirometry and 6-min walking test (6MWT) were reported to be independent predictors of mortality28,29. Additionally, an overestimated risk for mortality with the GAP index was reported by the same authors who developed the GAP index30. They reported improvement in risk prediction performance using a modified GAP index that also included longitudinal change in FVC and respiratory hospitalization30. However, assessing longitudinal variables and 6MWT may not be suitable for risk assessment in severe patients or patients for whom there are no historical data available.
In this setting, as the BMI was shown to have prognostic value independent of the GAP index and it is easy to measure, the present study examined the value of using GAP plus BMI in patients with IPF treated with AFT for prognostic separations. Recently, clinical implications of sarcopenia, a metabolic dysfunction involving loss of skeletal muscle, in ILD was reported beyond those in chronic obstructive pulmonary disease and cancer17,18,19. Indeed, skeletal-muscle loss was well correlated with lower BMI and was associated with worse outcome in patients with IPF18,19. Thus, lower BMI partly represents skeletal-muscle wasting in patients with IPF. Further, consistent with our findings, a second analysis of the INPULSIS study reported that lower BMI (< 25 kg/m2) and weight loss (> 5% during 52 weeks) were associated with faster decline in FVC, suggesting shorter survivals in such patients20. Although GAP plus BMI did not achieve significant improvement of model discrimination compared with that of the original GAP index, GAP plus BMI distinguished patients into four distinct prognoses. Especially, patients with GAP stages I and II (approximately 85% of our cohort) were re-classified from two groups into three groups. This multidimension approach might be helpful for physicians in practice.
The present study had several limitations. Although we confirmed the utility of the GAP index at AFT initiation using two cohorts of patients with IPF, this study was retrospective. Further, there had been reported concerns regarding the use of p-value in net reclassification improvement and its validity31. Second, the sample size was not sufficiently large. Given the small number of patients in each cohort, significance of GAP plus BMI was ascertained when we examined the two cohorts together. Third, the cut-off of the BMI appears to depend on ethnicity and country20. In our cohort, although few patients were obese, reverse-J associations were found between BMI and mortality, suggesting that both upper and lower cut-offs in BMI might be needed. Indeed, sarcopenic obesity is known to lead to poor outcomes in patients with cancer32. Collectively, these limitations may have introduced potential biases to this study. Thus, large-scale prospective studies are required to overcome these limitations.
In conclusion, the present retrospective study showed that assessment of the GAP index at AFT initiation could successfully separate patients with IPF in prognostic groups. Our multivariate prognostic evaluation also revealed that lower BMI was associated with poor outcome independent of the GAP index. Interestingly, GAP plus BMI also separated patients into four distinct prognoses in the combined-cohort of patients. Collectively, these results indicated the value of the GAP index and BMI measurement for assessing prognostic prediction in patients with IPF treated with AFT.
Methods
Subjects
This retrospective study initially included 311 consecutive patients with ILD who started treatment with pirfenidone or nintedanib at Hamamatsu University of School of Medicine (Hamamatsu cohort, n = 154), Seirei Hamamatsu Hospital, and Seirei Mikatahara Hospital (Seirei cohort, n = 157). All patients were treated between February 2009 and March 2020. Eighty-two patients with ILD were excluded from the study: 49 patients were diagnosed with non-IPF ILD, ten patients with IPF did not undergo spirometry, and 23 were not evaluated with the diffusion capacity of the lung for carbon monoxide test (DLCO) at the time of AFT initiation. Thus, this study finally included 229 patients with IPF treated with AFT who had available data to assess the GAP score: the Hamamatsu cohort (n = 110) and Seirei cohort (n = 119) (Fig. 1). All participants fulfilled the IPF consensus criteria1,25. The study protocol was approved by the Ethical Committee of Hamamatsu University School of Medicine (17-196) and was carried out in accordance with the approved guidelines. The need for patient approval and/or informed consent was waived by the Ethical Committee of Hamamatsu University School of Medicine, because of the retrospective nature of the study.
Data collection
Clinical data were obtained from the patients’ medical records. Laboratory findings and pulmonary and functional test results obtained at the time of AFT initiation were recorded. AE was diagnosed based on the ATS guidelines27,33.
Assessing the GAP index and GAP plus BMI index
The GAP score was calculated based on data at the time of AFT initiation according to a previous study3: sex (female, 0 points; male, 1 point), age (≤ 60 years , 0 points; 61–65 years, 1 point; > 65 years, 2 points), %FVC (> 75%, 0 points; 50–75%, 1 point; < 50%, 2 points), and %DLCO (> 55%, 0 points; 36–55%, 1 point; ≤ 35%, 2 points; cannot perform, 3 points). The GAP stage was defined based on the total GAP score: stage I (0–3 points), stage II (4–5 points), and stage III (6–8 points). The GAP plus BMI was calculated based on the GAP stage and BMI at the time of AFT initiation. If the BMI value was lower than the cut-off value, the severity of the GAP stage was advanced by one stage.
Statistical analysis
Discrete variables are expressed as totals (percentages), and continuous variables are expressed as the median [interquartile range]. The Mann–Whitney U test was used to compare continuous variables. Fisher’s exact test for independence was used to compare categorical variables. The area under the receiver operating characteristic (ROC) curve was used to identify the optimal cut-off for the BMI. The overall survival time was measured from AFT initiation. Univariate and multivariate analyses were also performed using a Cox proportional hazards regression model. Cumulative survival probabilities were calculated using the Kaplan–Meier method and the log-rank test. The model performance was evaluated by discrimination using concordance statistics (C-statistics), i.e., the ability of a model to discriminate those with an outcome from those without an outcome. Model improvement was calculated by comparison with the GAP index; change in C-statistics, integrated discrimination improvement (IDI), and net reclassification improvement (NRI) were employed. Statistical analyses were performed using JMP (Ver13, SAS Institute, Inc., Cary, NC) and R (Ver4.0.2, R Foundation for Statistical Computing, Vienna, Austria). All analyses were two-tailed, and P-values < 0.05 were considered statistically significant.
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Abbreviations
- AFT:
-
Antifibrotic therapy
- BMI:
-
Body mass index
- DLCO:
-
Diffusion capacity of the lung for carbon monoxide
- FVC:
-
Forced vital capacity
- GAP:
-
Gender-Age-Physiology
- IDI:
-
Integrated discrimination improvement
- ILD:
-
Interstitial lung disease
- IPF:
-
Idiopathic pulmonary fibrosis
- NRI:
-
Net reclassification improvement
- ROC:
-
Receiver operating characteristic
References
Raghu, G. et al. Diagnosis of idiopathic pulmonary fibrosis: An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 198, e44–e68. https://doi.org/10.1164/rccm.201807-1255ST (2018).
Raghu, G. et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: Treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am. J. Respir. Crit. Care Med. 192, e3–e19. https://doi.org/10.1164/rccm.201506-1063ST (2015).
Ley, B. et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann. Intern. Med. 156, 684–691. https://doi.org/10.7326/0003-4819-156-10-201205150-00004 (2012).
King, T. E. Jr. et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 370, 2083–2092. https://doi.org/10.1056/NEJMoa1402582 (2014).
Noble, P. W. et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): Two randomised trials. Lancet 377, 1760–1769. https://doi.org/10.1016/s0140-6736(11)60405-4 (2011).
Richeldi, L. et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N. Engl. J. Med. 365, 1079–1087. https://doi.org/10.1056/NEJMoa1103690 (2011).
Richeldi, L. et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 370, 2071–2082. https://doi.org/10.1056/NEJMoa1402584 (2014).
Distler, O. et al. Nintedanib for systemic sclerosis-associated interstitial lung disease. N. Engl. J. Med. 380, 2518–2528. https://doi.org/10.1056/NEJMoa1903076 (2019).
Flaherty, K. R. et al. Nintedanib in progressive fibrosing interstitial lung diseases. N. Engl. J. Med. 381, 1718–1727. https://doi.org/10.1056/NEJMoa1908681 (2019).
Maher, T. M. et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir. Med. 8, 147–157. https://doi.org/10.1016/s2213-2600(19)30341-8 (2020).
Dempsey, T. M. et al. Clinical effectiveness of antifibrotic medications for idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 200, 168–174. https://doi.org/10.1164/rccm.201902-0456OC (2019).
Nathan, S. D. et al. Effect of pirfenidone on mortality: Pooled analyses and meta-analyses of clinical trials in idiopathic pulmonary fibrosis. Lancet Respir. Med. 5, 33–41. https://doi.org/10.1016/s2213-2600(16)30326-5 (2017).
Alakhras, M., Decker, P. A., Nadrous, H. F., Collazo-Clavell, M. & Ryu, J. H. Body mass index and mortality in patients with idiopathic pulmonary fibrosis. Chest 131, 1448–1453. https://doi.org/10.1378/chest.06-2784 (2007).
Cruz-Jentoft, A. J. et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 48, 16–31. https://doi.org/10.1093/ageing/afy169 (2019).
Cruz-Jentoft, A. J. & Sayer, A. A. Sarcopenia. Lancet 393, 2636–2646. https://doi.org/10.1016/s0140-6736(19)31138-9 (2019).
Akahori, D. et al. Body composition changes successfully classify prognosis in patients with mycobacterium avium complex lung disease. J. Infect. 79, 341–348. https://doi.org/10.1016/j.jinf.2019.07.014 (2019).
Suzuki, Y. et al. Disease course and prognosis of pleuroparenchymal fibroelastosis compared with idiopathic pulmonary fibrosis. Respir. Med. https://doi.org/10.1016/j.rmed.2020.106078 (2020).
Suzuki, Y. et al. Distinct profile and prognostic impact of body composition changes in idiopathic pulmonary fibrosis and idiopathic pleuroparenchymal fibroelastosis. Sci. Rep. https://doi.org/10.1038/s41598-018-32478-z (2018).
Suzuki, Y. et al. Cause of mortality and sarcopenia in patients with idiopathic pulmonary fibrosis receiving antifibrotic therapy. Respirology (Carlton, VIC) 26, 171–179. https://doi.org/10.1111/resp.13943 (2021).
Jouneau, S. et al. Analysis of body mass index, weight loss and progression of idiopathic pulmonary fibrosis. Respir. Res. 21, 312. https://doi.org/10.1186/s12931-020-01528-4 (2020).
Kulkarni, T. et al. Decrements of body mass index are associated with poor outcomes of idiopathic pulmonary fibrosis patients. PLoS ONE 14, e0221905. https://doi.org/10.1371/journal.pone.0221905 (2019).
Raghu, G., Anstrom, K. J., King, T. E. Jr., Lasky, J. A. & Martinez, F. J. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N. Engl. J. Med. 366, 1968–1977. https://doi.org/10.1056/NEJMoa1113354 (2012).
Hyldgaard, C., Bendstrup, E., Wells, A. U. & Hilberg, O. Unclassifiable interstitial lung diseases: Clinical characteristics and survival. Respirology (Carlton, VIC) 22, 494–500. https://doi.org/10.1111/resp.12931 (2017).
Ryerson, C. J. et al. Predicting survival across chronic interstitial lung disease: The ILD-GAP model. Chest 145, 723–728. https://doi.org/10.1378/chest.13-1474 (2014).
Raghu, G. et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 183, 788–824. https://doi.org/10.1164/rccm.2009-040GL (2011).
Natsuizaka, M. et al. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am. J. Respir. Crit. Care Med. 190, 773–779. https://doi.org/10.1164/rccm.201403-0566OC (2014).
Collard, H. R. et al. Acute exacerbation of idiopathic pulmonary fibrosis. An international working group report. Am. J. Respir. Crit. Care Med. 194, 265–275. https://doi.org/10.1164/rccm.201604-0801CI (2016).
du Bois, R. M. et al. 6-Minute walk distance is an independent predictor of mortality in patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 43, 1421–1429. https://doi.org/10.1183/09031936.00131813 (2014).
du Bois, R. M. et al. Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 184, 459–466. https://doi.org/10.1164/rccm.201011-1790OC (2011).
Ley, B. et al. Unified baseline and longitudinal mortality prediction in idiopathic pulmonary fibrosis. Eur. Respir. J. 45, 1374–1381. https://doi.org/10.1183/09031936.00146314 (2015).
Pepe, M. S., Janes, H. & Li, C. I. Net risk reclassification p values: Valid or misleading?. J. Natl. Cancer Inst. 106, dju041. https://doi.org/10.1093/jnci/dju041 (2014).
Martin, L. et al. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 31, 1539–1547. https://doi.org/10.1200/JCO.2012.45.2722 (2013).
Collard, H. R. et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 176, 636–643. https://doi.org/10.1164/rccm.200703-463PP (2007).
Acknowledgements
This work is supported by a grant-in-aid for scientific research (19K17632 to Y.S.) from the Japan Society for the Promotion of Science. We thank Editage for editing a draft of the manuscript.
Author information
Authors and Affiliations
Contributions
Y.S.: Conception and design, data collection, data analysis and interpretation, manuscript writing, and final approval of the manuscript. M.K.: Data collection and data analysis. Y.A., K.M., H.H., and K.Y.: Conception and design, data collection, and data analysis. H.N., H.H., M.K., K.F., N.E., T.F., Y.N., N.I., and H.N.: Data collection, data analysis, and supervision. T.S.: Conception and design, manuscript writing, and administrative support.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suzuki, Y., Mori, K., Aono, Y. et al. Combined assessment of the GAP index and body mass index at antifibrotic therapy initiation for prognosis of idiopathic pulmonary fibrosis. Sci Rep 11, 18579 (2021). https://doi.org/10.1038/s41598-021-98161-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-98161-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.