Abstract
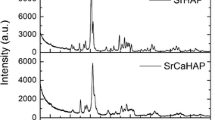
Adsorption behavior of lutetium by hydroxyapatite (HAP) was investigated by considering pH, adsorbent dose, contact time, initial concentration of lutetium, and temperature. The adsorbent was synthesized and characterized by X-ray diffraction (XRD; JCPDS file 01–04-3708), and the point of zero charge was 7.22. When the initial pH (pHi) was 3, the final pH (pHf) of the system was approximately 6, and in these conditions, approximately 98% of lutetium is present as Lu3+. The equilibrium of the adsorption system was reached in 5 min, and the HAP retained 99.2 ± 0.3% of lutetium. The experimental kinetic and isotherm data were adjusted to the pseudo-second-order and Freundlich models, respectively, indicating a chemisorption mechanism on a heterogeneous surface. Experimental and literature data revealed that Freundlich parameters depend on the cation size of the lanthanide elements; Kf (Freundlich constant) values linearly decrease with the ionic radii of the elements. The adsorption process was exothermic and spontaneous, as indicated by the negative values of enthalpy and Gibbs free energy values. The entropy value is small and negative, which may indicate that the affinity between Lu3+ and HPA is not strong.






taken from Granados-Correa et al. (2013) and the present work. The equations of the lines are Kf = 5.94 ionic radii (Å) + 8.03 (R2 = 0.999) and 1/n = 3.95 ionic radii (Å) -2.34


Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Audi, G., Kondev, F. G., Wang, M., Huang, W. J., & Naimi, S. (2017). The NUBASE 2016 evaluation of nuclear properties. Chinese Physics C, 41(3), 030001.
Awual, M. R., Alharthi, N. H., Okamoto, Y., Karim, M. R., Halim, M. E., Hasan, M. M., Rahman, M. M., Islam, M. M., Khaleque, M. A., & Sheikh, M. C. (2017). Ligand field effect for dysprosium(III) and lutetium(III) adsorption and EXAFS coordination with novel composite nanomaterials. Chemical Engineering Journal, 320, 427–435.
Banerjee, S., Pillai, M. R. A., & Knapp, F. F. (2015). Lutetium-177 therapeutic radiopharmaceuticals: Linking chemistry, radiochemistry, and practical applications. Chemical Reviews, 115(8), 2934–2974.
Castor, S. B. & Hedrick, J. B. (2006). Rare Earth Elements. In: J. Elzea Kogel, N. C. Trivedi, and J. M. Barker (Ed.). Industrial minerals and rocks. Society for Mining, Metallurgy and Exploration, 769–792.
Cawthray, J. F., Creagh, A. L., Haynes, C. A., & Orvig, C. (2015). Ion exchange in hydroxyapatite with lanthanides. Inorganic Chemistry, 54(4), 1440–1445.
Chakraborty, S., Das, T., Sarma, H. D., Venkatesh, M., & Banerjee, S. (2008). Preparation and preliminary studies on 177Lu-labeled hydroxyapatite particles for possible use in the therapy of liver cancer. Nuclear Medicine and Biology, 35(5), 589–597.
Chakraborty, S., Vimalnath, K. V., Rajeswari, A., Shinto, A., Sarma, H. D., Kamaleshwaran, K., & Dash, A. (2014). Preparation, evaluation, and first clinical use of 177Lu-labeled hydroxyapatite (HA) particles in the treatment of rheumatoid arthritis: Utility of cold kits for convenient dose formulation at hospital radiopharmacy. Journal of Labelled Compounds Radiopharmaceuticals, 57(7), 453–462.
Dada, A. O., Olalekan, A. P., Olatunya, A. M., & Dada, O. J. I. J. C. (2012). Langmuir, Freundlich, Temkin and Dubinin-Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR Journal of Applied Chemistry, 3(1), 38–45.
Granados-Correa, F., Vilchis-Granados, J., Jiménez-Reyes, M. & Quiroz-Granados, L. A. (2013). Adsorption behaviour of La (III) and Eu (III) ions from aqueous solutions by hydroxyapatite: Kinetic, isotherm, and thermodynamic studies. Journal of Chemistry, ID 751696, pp 1-9.
Haley, T. J. (1979). Toxicity. In: Gschneidner K. A. Jr. and Eyring L. R. (Eds.) Handbook on the physics and chemistry of the rare earths, Elsevier. Volume 4, Chapter 40, pp 553–585.
Ho, Y. S. (2006). Second-order kinetic model for the sorption of cadmium onto tree fern: A comparison of linear and non-linear methods. Water Research, 40(1), 119–125.
Jiménez-Reyes, M., & Solache-Ríos, M. (2010). Sorption behavior of fluoride ions from aqueous solutions by hydroxyapatite. Journal of Hazardous Materials, 180(1–3), 297–302.
Jiménez-Reyes, M., Almazán-Sánchez, P. T., & Solache-Ríos, M. (2020). Behaviour of cerium(III) in the presence of components of soils and its humate complex. Environmental Technology. https://doi.org/10.1080/09593330.2020.1758219
Kay, M. I., Young, R. A., & Posner, A. S. (1964). Crystal structure of hydroxyapatite. Nature, 204(4963), 1050–1052.
Keeling, A. A., & Vaughan, A. T. (1988). Factors influencing the adsorption of Lutetium-177 on hydroxyapatite. International Journal of Radiation Applications and Instrumentation. Part B. Nuclear Medicine and Biology, 15(5), 489–492.
Krestou, A., Xenidis, A., & Panias, D. (2004). Mechanism of aqueous uranium (VI) uptake by hydroxyapatite. Minerals Engineering, 17(3), 373–381.
Kegl, T., Košak, A., Lobnik, A., Novak, Z., Kralj, A. K., & Ban, I. (2020). Adsorption of rare earth metals from wastewater by nanomaterials: a review. Journal of Hazardous Materials, 386, 121632.
Li, J., Gong, A., Li, F., Qiu, L., Zhang, W., Gao, G., Liu, Y., & Li, J. (2018). Synthesis and characterization of magnetic mesoporous Fe3O4@mSiO2–DODGA nanoparticles for adsorption of 16 rare earth elements. RSC Advances, 8(68), 39149–39161.
Mobasherpour, I., Salahi, E., & Pazouki, M. (2012). Comparative of the removal of Pb2+, Cd2+ and Ni2+ by nano crystallite hydroxyapatite from aqueous solutions: Adsorption isotherm study. Arabian Journal of Chemistry, 5(4), 439–446.
Nayak, A. K. (2010). Hydroxyapatite synthesis methodologies: An overview. International Journal of ChemTech Research, 2(2), 903–907.
Pillai, M. R. A., & Knapp, F. F. (2015). Evolving important role of lutetium-177 for therapeutic nuclear medicine. Current Radiopharmaceuticals, 8(2), 78–85.
Puigdomenech, I. (2015). SPANA program. www.kth.se/che/medusasites/google.com/site/chemdiagr/
Roveri, N., & Palazzo, B. (2007). Hydroxyapatite nanocrystals as bone tissue substitute. Nanotechnologies for the Life Sciences, 9(Chapter 7), 283–307.
Saha, P. & Chowdhury, S. (2011). Insight into adsorption thermodynamics. Thermodynamics. https://www.semanticscholar.org/paper/Insight-Into-Adsorption-Thermodynamics-Saha-Chowdhury/b34f4bfea6e82a29c4d3530428635f0c5f6b37d9. Accessed July 2021.
Sebei, H., Pham Minh, D., Lyczko, N., Sharrock, P., & Nzihou, A. (2017). Hydroxyapatite-based sorbents: Elaboration, characterization, and application for the removal of catechol from the aqueous phase. Environmental Technology, 38(20), 2611–2620.
Skwarek, E., Gładysz-Płaska, A., & Bolbukh, Y. (2017). Adsorption of uranyl ions at the nano-hydroxyapatite and its modification. Nanoscale Research Letters, 12(1), 278.
Stechynska, E., Vasylechko, V., Gryshchouk, G., & Patsay, I. (2020). Preconcentration of lutetium from aqueous solution by Transcarpathian clinoptilolite. Acta Chimica Slovenica, 67(1), 105–112.
Trujillo-Nolasco, R. M., Morales-Avila, E., Ocampo-García, B. E., Ferro-Flores, G., Gibbens-Bandala, B. V., Escudero-Castellanos, A., & Isaac-Olive, K. (2019). Preparation and in vitro evaluation of radiolabeled HA-PLGA nanoparticles as novel MTX delivery system for local treatment of rheumatoid arthritis. Materials Science and Engineering: C, 103, 109766.
Funding
Dr. Almazán-Sánchez is supported by Catedras-CONACYT Fellowship – 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiménez-Reyes, M., Almazán-Sánchez, P.T., Jiménez-Becerril, J. et al. Adsorption of 177Lu from Water by Using Synthetic Hydroxyapatite. Water Air Soil Pollut 232, 394 (2021). https://doi.org/10.1007/s11270-021-05339-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05339-1