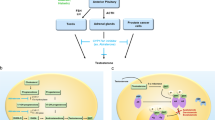
Abstract
Patients with high-risk prostate cancer treated with curative intent are at an increased risk of biochemical recurrence, metastatic progression and cancer-related death compared with patients treated for low-risk or intermediate-risk disease. Thus, these patients often need multimodal therapy to achieve complete disease control. Over the past two decades, multiple studies on the use of neoadjuvant treatment have been performed using conventional androgen deprivation therapy, which comprises luteinizing hormone-releasing hormone agonists or antagonists and/or first-line anti-androgens. However, despite results from these studies demonstrating a reduction in positive surgical margins and tumour volume, no benefit has been observed in hard oncological end points, such as cancer-related death. The introduction of potent androgen receptor signalling inhibitors (ARSIs), such as abiraterone, apalutamide, enzalutamide and darolutamide, has led to a renewed interest in using neoadjuvant hormonal treatment in high-risk prostate cancer. The addition of ARSIs to androgen deprivation therapy has demonstrated substantial survival benefits in the metastatic castration-resistant, non-metastatic castration-resistant and metastatic hormone-sensitive settings. Intuitively, a similar survival effect can be expected when applying ARSIs as a neoadjuvant strategy in high-risk prostate cancer. Most studies on neoadjuvant ARSIs use a pathological end point as a surrogate for long-term oncological outcome. However, no consensus yet exists regarding the ideal definition of pathological response following neoadjuvant hormonal therapy and pathologists might encounter difficulties in determining pathological response in hormonally treated prostate specimens. The neoadjuvant setting also provides opportunities to gain insight into resistance mechanisms against neoadjuvant hormonal therapy and, consequently, to guide personalized therapy.
Key points
-
Patients with high-risk prostate cancer are at an increased risk of biochemical recurrence, metastatic progression and cancer-related death following primary treatment compared with patients with low-risk or intermediate-risk disease.
-
Multiple studies on neoadjuvant treatment using conventional androgen deprivation therapy before radical prostatectomy have reported reduced positive surgical margins and tumour volume but no benefits in hard oncological end points.
-
Androgen receptor signalling inhibitors have demonstrated substantial survival benefits in metastatic and non-metastatic castration-resistant and metastatic hormone-sensitive prostate cancer.
-
The importance of pathological response following neoadjuvant hormonal therapy is unclear, and the effect of hormonal therapy on the prostate tissue renders pathological assessment difficult.
-
The presence of castration-resistant prostate cancer cells in early disease stages or rapid therapy-induced resistance might be responsible for the low proportion of pathological complete response (10%) in neoadjuvant androgen receptor signalling inhibitor studies.
-
Longitudinal imaging could help to identify early responders by assessing the effect of neoadjuvant hormonal therapy on the local tumour and possible micrometastases.
-
Ongoing and future neoadjuvant studies should include translational end points to identify early resistance mechanisms and to develop novel biomarkers, enabling personalized neoadjuvant hormonal treatment for patients with high-risk prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Cooperberg, M. R., Broering, J. M. & Carroll, P. R. Time trends and local variation in primary treatment of localized prostate cancer. J. Clin. Oncol. 28, 1117–1123 (2010).
Zelic, R. et al. Predicting prostate cancer death with different pretreatment risk stratification tools: a head-to-head comparison in a nationwide cohort study. Eur. Urol. 77, 180–188 (2020).
Joniau, S. et al. Stratification of high-risk prostate cancer into prognostic categories: a European multi-institutional study. Eur. Urol. 67, 157–164 (2015).
D’Amico, A. V. et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280, 969–974 (1998).
Sanda, M. G. et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part I: risk stratification, shared decision making, and care options. J. Urol. 199, 683–690 (2018).
Dasgupta, P., Davis, J. & Hughes, S. NICE guidelines on prostate cancer 2019. BJU Int. 124, 1 (2019).
Mottet, N. et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer — 2020 Update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur. Urol. 79, 243–262 (2021).
Albertsen, P. C., Hanley, J. A. & Fine, J. 20-Year outcomes following conservative management of clinically localized prostate cancer. JAMA 293, 2095 (2005).
Akre, O. et al. Mortality among men with locally advanced prostate cancer managed with noncurative intent: a nationwide study in PCBaSe Sweden. Eur. Urol. 60, 554–563 (2011).
Rider, J. R. et al. Long-term outcomes among noncuratively treated men according to prostate cancer risk category in a nationwide, population-based study. Eur. Urol. 63, 88–96 (2013).
Moris, L. et al. Benefits and risks of primary treatments for high-risk localized and locally advanced prostate cancer: an international multidisciplinary systematic review. Eur. Urol. 77, 614–627 (2020).
Van den Broeck, T. et al. Prognostic value of biochemical recurrence following treatment with curative intent for prostate cancer: a systematic review. Eur. Urol. 75, 967–987 (2018).
Briganti, A. et al. Natural history of surgically treated high-risk prostate cancer. Urol. Oncol. Semin. Orig. Investig. 33, 163.e7–163.e13 (2015).
Jackson, W. C. et al. Intermediate endpoints after postprostatectomy radiotherapy: 5-year distant metastasis to predict overall survival. Eur. Urol. 74, 413–419 (2018).
Bolla, M. et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: Long-term results of a randomised controlled trial (EORTC trial 22911). Lancet 380, 2018–2027 (2012).
Kent, E. C. & Hussain, M. H. Neoadjuvant therapy for prostate cancer: an Oncologist’s perspective. Rev. Urol. 5, S28–S37 (2003).
Petrelli, F. et al. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: a meta-analysis. Eur. Urol. 65, 350–357 (2014).
Berger, A. C. et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J. Clin. Oncol. 23, 4330–4337 (2005).
Amiri-Kordestani, L. et al. First FDA approval of neoadjuvant therapy for breast cancer: Pertuzumab for the treatment of patients with HER2-positive breast cancer. Clin. Cancer Res. 20, 5359–5364 (2014).
Kidane, B., Coughlin, S., Vogt, K. & Malthaner, R. Preoperative chemotherapy for resectable thoracic esophageal cancer. Cochrane Database of Syst. Rev. 2015, CD001556 (2015).
Crawford, E. D. et al. Androgen-targeted therapy in men with prostate cancer: evolving practice and future considerations. Prostate Cancer Prostatic Dis. 22, 24–38 (2018).
Bianco, F. J. et al. Proliferation of prostate cancer cells in the bone marrow predicts recurrence in patients with localized prostate cancer. Prostate 49, 235–242 (2001).
Wood, D. P. & Banerjee, M. Presence of circulating prostate cells in the bone marrow of patients undergoing radical prostatectomy is predictive of disease-free survival. J. Clin. Oncol. 15, 3451–3457 (1997).
Köllermann, J. et al. Prognostic significance of disseminated tumor cells in the bone marrow of prostate cancer patients treated with neoadjuvant hormone treatment. J. Clin. Oncol. 26, 4928–4933 (2008).
Cher, M. L. et al. Cellular proliferation and prevalence of micrometastatic cells in the bone marrow of patients with clinically localized prostate cancer. Clin. Cancer Res. 5, 2421–2425 (1999).
Morgan, T. M. et al. Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clin. Cancer Res. 15, 677–683 (2009).
Melchior, S. W. et al. Early tumor cell dissemination in patients with clinically localized carcinoma of the prostate. Clin. Cancer Res. 3, 249–256 (1997).
Berg, A. et al. Impact of disseminated tumor cells in bone marrow at diagnosis in patients with nonmetastatic prostate cancer treated by definitive radiotherapy. Int. J. Cancer 120, 1603–1609 (2007).
Davey, R. A. & Grossmann, M. Androgen receptor structure, function and biology: from bench to bedside. Clin. Biochem. Rev. 37, 3 (2016).
Dai, C., Heemers, H. & Sharifi, N. Androgen signaling in prostate cancer. Cold Spring Harb. Perspect. Med. 7, a030452 (2017).
Mostaghel, E. A. Steroid hormone synthetic pathways in prostate cancer. Transl Androl. Urol. 2, 212–227 (2013).
Mostaghel, E. A. & Nelson, P. S. Intracrine androgen metabolism in prostate cancer progression: mechanisms of castration resistance and therapeutic implications. Best Pract. Res.Clin. Endocrinol. Metab. 22, 243–258 (2008).
Cattrini, C. et al. Current treatment options for metastatic hormone-sensitive prostate cancer. Cancers 11, 1355 (2019).
Wadosky, K. M. & Koochekpour, S. Therapeutic rationales, progresses, failures, and future directions for advanced prostate cancer. Int. J. Biol. Sci. 12, 409–426 (2016).
Ku, S. Y., Gleave, M. E. & Beltran, H. Towards precision oncology in advanced prostate cancer. Nat. Rev. Urol. 16, 645–654 (2019).
Tran, C. et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324, 787–790 (2009).
Rice, M. A., Malhotra, S. V. & Stoyanova, T. Second-generation antiandrogens: from discovery to standard of care in castration resistant prostate cancer. Front. Oncol. 10, 801 (2019).
Ryan, C. J. et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): Final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 16, 152–160 (2015).
de Bono, J. S. et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 364, 1995–2005 (2011).
Beer, T. M. et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 371, 424–433 (2014).
Scher, H. I. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 367, 1187–1197 (2012).
Smith, M. R. et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N. Engl. J. Med. 378, 1408–1418 (2018).
Hussain, M. et al. PROSPER: a phase 3, randomized, double-blind, placebo (PBO)-controlled study of enzalutamide (ENZA) in men with nonmetastatic castration-resistant prostate cancer (M0 CRPC). J. Clin. Oncol. 36, 3–3 (2018).
Fizazi, K. et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N. Engl. J. Med. 380, 1235–1246 (2019).
Fizazi, K. et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Artic. Lancet Oncol. 20, 686–700 (2019).
Armstrong, A. J. et al. Arches: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol 37, 2974–2986 (2019).
Chi, K. N. et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N. Engl. J. Med. 381, 13–24 (2019).
Carceles-Cordon, M. et al. Cellular rewiring in lethal prostate cancer: the architect of drug resistance. Nat. Rev. Urol. 17, 292–307 (2020).
Goldie, J. H. & Coldman, A. J. A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat. Rep. 63, 1727–1733 (1979).
Goldie, J. H. Mathematical models of drug resistance and chemotherapy effects. Cancer Treat. Res. 48, 13–26 (1989).
Bolla, M. et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 11, 1066–1073 (2010).
Iversen, P. et al. Antiandrogen monotherapy in patients with localized or locally advanced prostate cancer: final results from the bicalutamide Early Prostate Cancer programme at a median follow-up of 9.7 years. BJU Int. 105, 1074–1081 (2010).
Bolla, M. et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): A phase III randomised trial. Lancet 360, 103–108 (2002).
See, W. A. & Tyrrell, C. J. The addition of bicalutamide 150 mg to radiotherapy significantly improves overall survival in men with locally advanced prostate cancer. J. Cancer Res. Clin. Oncol. 132, 7–16 (2006).
Denham, J. W. et al. Short-term androgen suppression and radiotherapy versus intermediate-term androgen suppression and radiotherapy, with or without zoledronic acid, in men with locally advanced prostate cancer (TROG 03.04 RADAR): 10-year results from a randomised, phase 3, factorial trial. Lancet Oncol. 20, 267–281 (2019).
Roach, M. et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J. Clin. Oncol. 26, 585–591 (2008).
Spratt, D. E. et al. Androgen receptor upregulation mediates radioresistance after ionizing radiation. Cancer Res. 75, 4688–4696 (2015).
Bartek, J., Mistrik, M. & Bartkova, J. Androgen receptor signaling fuels DNA repair and radioresistance in prostate cancer. Cancer Discov. 3, 1222–1224 (2013).
Polkinghorn, W. R. et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 3, 1245–1253 (2013).
Boevé, L. M. S. et al. Effect on survival of androgen deprivation therapy alone compared to androgen deprivation therapy combined with concurrent radiation therapy to the prostate in patients with primary bone metastatic prostate cancer in a prospective randomised clinical trial: data from the HORRAD trial. Eur. Urol. 75, 410–418 (2019).
Parker, C. C. et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet 392, 2353–2366 (2018).
Burdett, S. et al. Prostate radiotherapy for metastatic hormone-sensitive prostate cancer: A STOPCAP systematic review and meta-analysis. Eur. Urol. 76, 115–124 (2019).
Sooriakumaran, P. et al. TRoMbone: Testing radical prostatectomy in men with oligo metastatic prostate cancer that has spread to the bone - a randomized controlled feasibility trial [abstract]. Eur. Urol. Suppl. 18, e2199 (2019).
Isbarn, H. et al. Androgen deprivation therapy for the treatment of prostate cancer: consider both benefits and risks. Eur. Urol. 55, 62–75 (2009).
Sonpavde, G. & Sternberg, C. N. Neoadjuvant systemic therapy for urological malignancies. BJU Int. 106, 6–22 (2009).
van der Kwast, T. H. et al. Prolonged neoadjuvant combined androgen blockade leads to a further reduction of prostatic tumor volume: three versus six months of endocrine therapy. Urology 53, 523–529 (1999).
Soloway, M. S. et al. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year results. J. Urol. 167, 112–116 (2002).
Fair, W. R. et al. The indications, rationale, and results of neoadjuvant androgen deprivation in the treatment of prostatic cancer: Memorial Sloan-Kettering Cancer Center results. Urology 49, 46–55 (1997).
Meyer, F. et al. Neoadjuvant hormonal therapy before radical prostatectomy and risk of prostate specific antigen failure. J. Urol. 162, 2024–2028 (1999).
Berglund, R. K. et al. Ten-year follow-up of neoadjuvant therapy with goserelin acetate and flutamide before radical prostatectomy for clinical T3 and T4 prostate cancer: update on Southwest Oncology Group Study 9109. Urology 79, 633–637 (2012).
Goldenberg, S. L. et al. Randomized, prospective, controlled study comparing radical prostatectomy alone and neoadjuvant androgen withdrawal in the treatment of localized prostate cancer. J. Urol. 156, 873–877 (1996).
Labrie, F. et al. Neoadjuvant hormonal therapy: the Canadian experience. Urology 49, 56–64 (1997).
Selli, C. et al. Effects of complete androgen blockade for 12 and 24 weeks on the pathological stage and resection margin status of prostate cancer. J. Clin. Pathol. 55, 508–513 (2002).
Gleave, M. E. et al. Randomized comparative study of 3 versus 8-month neoadjuvant hormonal therapy before radical prostatectomy: biochemical and pathological effects. J. Urol. 166, 500–507 (2001).
Prezioso, D., Lotti, T., Polito, M. & Montironi, R. Neoadjuvant hormone treatment with leuprolide acetate depot 3.75 mg and Cyproterone acetate, before radical prostatectomy: a randomized study. Urol. Int. 72, 189–195 (2004).
Dalkin, B. L., Ahmann, F. R., Nagle, R. & Johnson, C. S. Randomized study of neoadjuvant testicular androgen ablation therapy before radical prostatectomy in men with clinically localized prostate cancer. J. Urol. 155, 1357–1360 (1996).
Klotz, L. H. et al. Long-term followup of a randomized trial of 0 versus 3 months of neoadjuvant androgen ablation before radical prostatectomy. J. Urol. 170, 791–794 (2003).
Van Poppel, H. et al. Neoadjuvant hormonal therapy before radical prostatectomy decreases the number of positive surgical margins in stage T2 prostate cancer: interim results of a prospective randomized trial. The Belgian Uro-Oncological Study Group. J.Urol. 154, 429–434 (1995).
Aus, G. et al. Three-month neoadjuvant hormonal therapy before radical prostatectomy: a 7-year follow-up of a randomized controlled trial. BJU Int. 90, 561–566 (2002).
Schulman, C. C. et al. 4-Year follow-up results of a European prospective randomized study on neoadjuvant hormonal therapy prior to radical prostatectomy in T2-3N0M0 prostate cancer. Eur. Urol. 38, 706–713 (2000).
Shelley, M. D. et al. A systematic review and meta-analysis of randomised trials of neo-adjuvant hormone therapy for localised and locally advanced prostate carcinoma. Cancer Treat. Rev. 35, 9–17 (2009).
Lou, D. Y. & Fong, L. Neoadjuvant therapy for localized prostate cancer: examining mechanism of action and efficacy within the tumor. Urol. Oncol. 34, 182–192 (2016).
Mostaghel, E. A. et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 67, 5033–5041 (2007).
Mostaghel, E. A. et al. Targeted androgen pathway suppression in localized prostate cancer: a pilot study. J. Clin. Oncol. 32, 229–237 (2014).
Gregory, C. W., Johnson, R. T., Mohler, J. L., French, F. S. & Wilson, E. M. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 61, 2892–2898 (2001).
McKay, R. R. et al. Evaluation of intense androgen deprivation before prostatectomy: a randomized phase II trial of enzalutamide and leuprolide with or without abiraterone. J. Clin. Oncol. 11, 1–10 (2019).
Montgomery, B. et al. Neoadjuvant enzalutamide prior to prostatectomy. Clin. Cancer Res. 23, 2169–2176 (2017).
Graham, L. S. et al. Targeting backdoor androgen synthesis through AKR1C3 inhibition: A presurgical hormonal ablative neoadjuvant trial in high-risk localized prostate cancer. Prostate 81, 418–426 (2021).
Taplin, M. E. et al. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a randomized phase II neoadjuvant study. J. Clin. Oncol. 32, 3705–3715 (2014).
Efstathiou, E. et al. Neoadjuvant apalutamide (APA) plus leuprolide (LHRHa) with or without abiraterone (AA) in localized high-risk prostate cancer (LHRPC). J. Clin.Oncol. 38, Abstr. 5504–5504 (2020).
Efstathiou, E. et al. Clinical and biological characterisation of localised high-risk prostate cancer: results of a randomised preoperative study of a luteinising hormone-releasing hormone agonist with or without abiraterone acetate plus prednisone. Eur. Urol. 76, 418–424 (2019).
Aslim, E. J. et al. Neoadjuvant apalutamide (arn-509) and radical prostatectomy in treatment of intermediate to high risk prostate cancer (NEAR) — initial results of a phase II trial [abstract]. Eur. Urol. Suppl. 18, e1394 (2019).
Corcoran, N. et al. The predictive value of ARv7 expression in localized prostate caner treated with abiraterone, degarelix, and bicalutamide. J. Clin. Oncol. 33, Abstr. 71 (2015).
McKay, R. R. et al. Results of a randomized phase II trial of intense androgen deprivation therapy prior to radical prostatectomy in men with high-risk localized prostate cancer. J. Urol. 206, 80–87 (2021).
Karzai, F. et al. Sequential prostate magnetic resonance imaging in newly diagnosed high-risk prostate cancer treated with neoadjuvant enzalutamide is predictive of therapeutic response. Clin. Cancer Res. 27, 429–437 (2021).
Wilkinson, S. et al. Nascent prostate cancer heterogeneity drives evolution and resistance to intense hormonal therapy. Eur. Urol. https://doi.org/10.1016/j.eururo.2021.03.009 (2021).
O’Donnell, A. et al. Hormonal impact of the 17α-hydroxylase/C17,20-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br. J. Cancer 90, 2317–2325 (2004).
Chen, M. E. et al. A streamlined three-dimensional volume estimation method accurately classifies prostate tumors by volume. Am. J. Surg. Pathol. 27, 1291–1301 (2003).
Symmans, W. F. et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. 25, 4414–4422 (2007).
Yang, X. Y. et al. Initial patient-reported outcomes of a phase II neoadjuvant apalutamide (ARN-509) and radical prostatectomy in treatment of intermediate to high risk prostate cancer (NEAR) trial [abstract]. Eur. Urol. Suppl. 18, e1402 (2019).
Tamae, D. et al. The DHEA-sulfate depot following P450c17 inhibition supports the case for AKR1C3 inhibition in high risk localized and advanced castration resistant prostate cancer. Chem. Biol. Interact. 234, 332–338 (2015).
Hofland, J. et al. Evidence of limited contributions for intratumoral steroidogenesis in prostate cancer. Cancer Res. 70, 1256–1264 (2010).
Locke, J. A. et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 68, 6407–6415 (2008).
Liu, C. et al. Intracrine androgens and AKR1C3 activation confer resistance to enzalutamide in prostate cancer. Cancer Res. 75, 1413–1422 (2015).
Sowalsky, A. G. et al. Neoadjuvant-intensive androgen deprivation therapy selects for prostate tumor foci with diverse subclonal oncogenic alterations. Cancer Res. 78, 4716–4730 (2018).
Deng, Q. & Tang, D. G. Androgen receptor and prostate cancer stem cells: Biological mechanisms and clinical implications. Endocr. Relat. Cancer 22, T209–T220 (2015).
Eigl, B. J. C. et al. Timing is everything: preclinical evidence supporting simultaneous rather than sequential chemohormonal therapy for prostate cancer. Clin. Cancer Res. 11, 4905–4911 (2005).
Fizazi, K. et al. Androgen deprivation therapy plus docetaxel and estramustine versus androgen deprivation therapy alone for high-risk localised prostate cancer (GETUG 12): a phase 3 randomised controlled trial. Lancet Oncol. 16, 787–794 (2015).
Rosenthal, S. A. et al. Effect of chemotherapy with docetaxel with androgen suppression and radiotherapy for localized high-risk prostate cancer: the randomized phase III NRG Oncology RTOG 0521 Trial. J. Clin. Oncol. 37, 1159–1168 (2019).
Kyriakopoulos, C. E. et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J. Clin. Oncol. 36, 1080–1087 (2018).
Thalgott, M. et al. Long-term results of a phase II study with neoadjuvant docetaxel chemotherapy and complete androgen blockade in locally advanced and high-risk prostate cancer. J. Hematol. Oncol. 7, 20 (2014).
Silberstein, J. L. et al. Long-term oncological outcomes of a phase II trial of neoadjuvant chemohormonal therapy followed by radical prostatectomy for patients with clinically localised, high-risk prostate cancer. BJU Int. 116, 50–56 (2015).
Koie, T. et al. Safety and effectiveness of neoadjuvant luteinizing hormone-releasing hormone agonist plus low-dose estramustine phosphate in high-risk prostate cancer: a prospective single-arm study. Prostate Cancer Prostatic Dis. 15, 397–401 (2012).
Narita, S. et al. Short-term clinicopathological outcome of neoadjuvant chemohormonal therapy comprising complete androgen blockade, followed by treatment with docetaxel and estramustine phosphate before radical prostatectomy in Japanese patients with high-risk localized prostate cancer. World J. Surg. Oncol. 10, 1 (2012).
Zurita, A. J. et al. Integrating chemohormonal therapy and surgery in known or suspected lymph node metastatic prostate cancer. Prostate Cancer Prostatic Dis. 18, 276–280 (2015).
Nosov, A. et al. Safety and efficacy of neoadjuvant chemohormonal and hormonal treatment followed by radical prostatectomy for patients with high- and very high risk prostate cancer: Initial results of prospective, randomized, phase III clinical trial [abstract]. Eur. Urol. Suppl. 15, e1193 (2016).
Chi, K. N. et al. Multicenter phase II study of combined neoadjuvant docetaxel and hormone therapy before radical prostatectomy for patients with high risk localized prostate cancer. J. Urol. 180, 565–570 (2008).
Eastham, J. A. et al. Cancer and Leukemia Group B 90203 (Alliance): radical prostatectomy with or without neoadjuvant chemohormonal therapy in localized, high-risk prostate cancer. J. Clin. Oncol. 38, 3042–3050 (2020).
Gharzai, L. A. et al. Intermediate clinical endpoints for surrogacy in localised prostate cancer: an aggregate meta-analysis. Lancet Oncol. 22, 402–410 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03436654 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/results/NCT03279250 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03821246 (2021).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT04356430 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02949284 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02903368 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02770391 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02789878 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03767244 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03080116 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02543255 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03297385 (2017).
Wilkinson, S. & Sowalsky, A. G. Battling the two-headed dragon of prostate cancer targeted therapy. Mol. Cell Oncol. 7, 1745037 (2020).
Mckay, R. R. et al. Post prostatectomy outcomes of patients with high-risk prostate cancer treated with neoadjuvant androgen blockade. Prostate Cancer Prostatic Dis. 21, 364–372 (2018).
McKay, R. R. et al. Outcomes post neoadjuvant intense hormone therapy and surgery for patients with high-risk localized prostate cancer: results of a pooled analysis of contemporary clinical trials. J. Urol. 205, 1689–1697 (2021).
Rödel, C. et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J. Clin. Oncol. 23, 8688–8696 (2005).
Grossman, H. B. et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N. Engl. J. Med. 349, 859–866 (2003).
Gianni, L. et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 13, 25–32 (2012).
Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384, 164–172 (2014).
Peintinger, F. et al. Reproducibility of residual cancer burden for prognostic assessment of breast cancer after neoadjuvant chemotherapy. Mod. Pathol. 28, 913–920 (2015).
Kollermann, J. et al. Prognosis of stage pT0 after prolonged neoadjuvant endocrine therapy of prostate cancer: a matched-pair analysis. Eur. Urol. 45, 42–45 (2004).
Barry, M., Perner, S., Demichelis, F. & Rubin, M. A. TMPRSS2-ERG fusion heterogeneity in multifocal prostate cancer: clinical and biologic implications. Urology 70, 630–633 (2007).
Meiers, I., Waters, D. J. & Bostwick, D. G. Preoperative prediction of multifocal prostate cancer and application of focal therapy: review 2007. Urology 70, 3–8 (2007).
Fraser, M. et al. Genomic hallmarks of localized, non-indolent prostate cancer. Nature 541, 359–364 (2017).
Espiritu, S. M. G. et al. The evolutionary landscape of localized prostate cancers drives clinical aggression. Cell 173, 1003–1013.e15 (2018).
Neri, A. et al. Clinical significance of multifocal and multicentric breast cancers and choice of surgical treatment: a retrospective study on a series of 1158 cases. BMC Surg. 15, 1–10 (2015).
Joergensen, L. E., Gunnarsdottir, K. A., Lanng, C., Moeller, S. & Rasmussen, B. B. Multifocality as a prognostic factor in breast cancer patients registered in Danish Breast Cancer Cooperative Group (DBCG) 1996–2001. Breast 17, 587–591 (2008).
Ogston, K. N. et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast 12, 320–327 (2003).
Smith, I. C. et al. Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J. Clin. Oncol. 20, 1456–1466 (2002).
Evans, A. J. Treatment effects in prostate cancer. Mod. Pathol. 31, 110–121 (2018).
Têtu, B. Morphological changes induced by androgen blockade in normal prostate and prostatic carcinoma. Best Pract. Res. 22, 271–283 (2008).
Thomsen, O. Ø. et al. A comparison of budesonide and mesalamine for active Crohn’s disease. N. Engl. J. Med. 339, 370–374 (1998).
Penault-Llorca, F. et al. Comparison of the prognostic significance of Chevallier and Sataloff’s pathologic classifications after neoadjuvant chemotherapy of operable breast cancer. Hum. Pathol. 39, 1221–1228 (2008).
Pinder, S. E. et al. Macroscopic handling and reporting of breast cancer specimens pre- and post-neoadjuvant chemotherapy treatment: review of pathological issues and suggested approaches. Histopathology 67, 279–293 (2015).
Pinder, S. E., Provenzano, E., Earl, H. & Ellis, I. O. Laboratory handling and histology reporting of breast specimens from patients who have received neoadjuvant chemotherapy. Histopathology 50, 409–417 (2007).
Epstein, J. I. et al. The 2005 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am. J. Surg. Pathol. 29, 1228–1242 (2005).
Efstathiou, E. et al. Morphologic characterization of preoperatively treated prostate cancer: toward a post-therapy histologic classification. Eur. Urol. 57, 1030–1038 (2010).
Murphy, C. et al. A novel system for estimating residual disease and pathologic response to neoadjuvant treatment of prostate cancer. Prostate 76, 1285–1292 (2016).
Dogdas, B. et al. Computational pathological identification of prostate cancer following neoadjuvant treatment. J. Clin. Oncol. 38, Abstr. e14052–e14052 (2020).
Gold, S. A. et al. mpMRI preoperative staging in men treated with antiandrogen and androgen deprivation therapy before robotic prostatectomy. Urol. Oncol. Semin. Orig. Investig. 37, 352.e25–352.e30 (2019).
Hövels, A. M. et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin. Radiol. 63, 387–395 (2008).
Maurer, T., Eiber, M., Schwaiger, M. & Gschwend, J. E. Current use of PSMA–PET in prostate cancer management. Nat. Rev. Urol. 13, 226–235 (2016).
Perera, M. et al. Sensitivity, specificity, and predictors of positive 68 Ga–prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur. Urol. 70, 926–937 (2016).
Calais, J. et al. 68Ga-PSMA-11 PET/CT mapping of prostate cancer biochemical recurrence after radical prostatectomy in 270 patients with a PSA level of less than 1.0 ng/ml: impact on salvage radiotherapy planning. J. Nucl. Med. 59, 230–237 (2018).
McCarthy, M., Francis, R., Tang, C., Watts, J. & Campbell, A. A multicenter prospective clinical trial of 68 gallium PSMA HBED-CC PET-CT restaging in biochemically relapsed prostate carcinoma: oligometastatic rate and distribution compared with standard imaging. Int. J. Radiat. Oncol. Biol. Phys. 104, 801–808 (2019).
Hofman, M. S. et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet 395, 1208–1216 (2020).
Tosco, L. et al. Neoadjuvant degarelix with or without apalutamide followed by radical prostatectomy for intermediate and high-risk prostate cancer: ARNEO, a randomized, double blind, placebo-controlled trial. BMC Cancer 18, 354 (2018).
Cho, S. et al. Preliminary analysis of PSMA-targeted 18F-DCFPyL PET/MRI for assessment of response to neo-adjuvant chemohormonal therapy in men with high-risk primary prostate cancer. J. Nucl. Med. 61, 1264 (2020).
Chen, M. et al. Can 68 Ga-PSMA-11 positorn emission technology/computerized tomography predict pathologic response of primary prostate cancer to neoadjuvant androgen deprivation therapy?: A Pilot Study. J. Urol. 205, 1082–1089 (2020).
Vaz, S. et al. Influence of androgen deprivation therapy on PSMA expression and PSMA-ligand PET imaging of prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 47, 9–15 (2020).
Batra, J. S., Pienta, K. J., Pomper, M. G., Gorin, M. A. & Rowe, S. P. Can the interplay between androgen signaling and PSMA expression be leveraged for theranostic applications? Transl Androl. Urol. 8, S263–S264 (2019).
Evans, M. J. et al. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proc. Natl Acad. Sci. USA 108, 9578–9582 (2011).
Spans, L. et al. The genomic landscape of prostate cancer. Int. J. Mol. Sci. 14, 10822–10851 (2013).
Devlies, W. et al. Clinical actionability of the genomic landscape of metastatic castration resistant prostate cancer. Cells 9, 2494 (2020).
Beltran, H. et al. Impact of therapy on genomics and transcriptomics in high-risk prostate cance treated with neoadjuvant docetaxel and androgen deprivation therapy. Clin. Cancer Res. 23, 6802–6811 (2017).
Handle, F. & Claessens, F. AR variants: lost in translation to clinical practice? Nat. Rev. Urol. 16, 451–452 (2019).
Antonarakis, E. S. et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 371, 1028–1038 (2014).
Arora, V. K. et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 155, 1309–1322 (2013).
Puhr, M. et al. The glucocorticoid receptor is a key player for prostate cancer cell survival and a target for improved antiandrogen therapy. Clin. Cancer Res. 24, 927–938 (2018).
Claessens, F., Joniau, S. & Helsen, C. Comparing the rules of engagement of androgen and glucocorticoid receptors. Cell. Mol. Life Sci. 74, 2217–2228 (2017).
Xie, N. et al. The expression of glucocorticoid receptor is negatively regulated by active androgen receptor signaling in prostate tumors. Int. J. Cancer 136, E27–E38 (2015).
Li, J. et al. Aberrant corticosteroid metabolism in tumor cells enables GR takeover in enzalutamide resistant prostate cancer. eLife 6, e20183 (2017).
Kaochar, S. & Mitsiades, N. Glucocorticoids mediate adverse events of deep androgen receptor axis inhibition in prostate cancer patients. Ann. Oncol. 31, 323–325 (2020).
Taplin, M. E. et al. A phase II study of mifepristone (RU-486) in castration-resistant prostate cancer, with a correlative assessment of androgen-related hormones. BJU Int. 101, 1084–1089 (2008).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02012296 (2018).
Gao, S. et al. ErbB2 signaling increases androgen receptor expression in abiraterone-resistant prostate cancer. Clin. Cancer Res. 22, 3672–3682 (2016).
Miller, D. R., Ingersoll, M. A. & Lin, M. F. ErbB-2 signaling in advanced prostate cancer progression and potential therapy. Endocr. Rel. Cancer 26, R195–R209 (2018).
Shiota, M. et al. Inhibition of the HER2-YB1-AR axis with lapatinib synergistically enhances enzalutamide anti-tumor efficacy in castration resistant prostate cancer. Oncotarget 6, 9086–9098 (2015).
Bishop, J. L. et al. PD-L1 is highly expressed in enzalutamide resistant prostate cancer. Oncotarget 6, 234–242 (2015).
Liu, J. et al. p300/CBP inhibition enhances the efficacy of programmed death-ligand 1 blockade treatment in prostate cancer. Oncogene 39, 3939–3951 (2020).
Lieping, C. & Xue, H. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J. Clin. Invest. 125, 3384–3391 (2015).
Calagua, C. et al. Expression of PD-L1 in hormone-naïve and treated prostate cancer patients receiving neoadjuvant abiraterone acetate plus prednisone and leuprolide. Clin. Cancer Res. 23, 6812–6822 (2017).
Sharma, A. et al. The retinoblastoma tumor suppressor controls androgen signaling and human prostate cancer progression. J. Clin. Invest. 120, 4478–4492 (2010).
Tan, H. L. et al. Rb loss is characteristic of prostatic small cell neuroendocrine carcinoma. Clin. Cancer Res. 20, 890–903 (2014).
Beltran, H. et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 22, 298–305 (2016).
Wyatt, A. W. et al. Genomic alterations in cell-free DNA and enzalutamide resistance in castration-resistant prostate cancer. JAMA Oncol. 2, 1598–1606 (2016).
Abida, W. et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc. Natl Acad. Sci. USA 116, 11428–11436 (2019).
Carver, B. S. et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 19, 575–586 (2011).
Mateo, J. et al. Genomics of lethal prostate cancer at diagnosis and castration-resistance. J. Clin. Invest. 130, 1743–1751 (2019).
Yu, J. et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell 17, 443–454 (2010).
De Laere, B. et al. TP53 outperforms other androgen receptor biomarkers to predict abiraterone or enzalutamide outcome in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 25, 1766–1773 (2019).
Aparicio, A. M. et al. Combined tumor suppressor defects characterize clinically defined aggressive variant prostate cancers. Clin. Cancer Res. 22, 1520–1530 (2016).
Castro, E. et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J. Clin. Oncol. 31, 1748–1757 (2013).
Marshall, C. H. et al. Prevalence of DNA repair gene mutations in localized prostate cancer according to clinical and pathologic features: association of Gleason score and tumor stage. Prostate Cancer Prostatic Dis. 22, 59–65 (2019).
Isaacsson Velho, P. et al. Molecular characterization and clinical outcomes of primary gleason pattern 5 prostate cancer after radical prostatectomy. JCO Precis. Oncol. 3, 1–13 (2019).
Isaacsson Velho, P. et al. Intraductal/ductal histology and lymphovascular invasion are associated with germline DNA-repair gene mutations in prostate cancer. Prostate 78, 401–407 (2018).
Berchuck, J. E. et al. Impact of pathogenic germline DNA damage repair alterations on response to intense neoadjuvant androgen deprivation therapy in high-risk localized prostate cancer. Eur. Urol. 80, 295–303 (2021).
Scher, H. I. et al. Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer. JAMA Oncol. 2, 1441–1449 (2016).
Antonarakis, E. S. et al. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 1, 582–591 (2015).
Le, D. T. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372, 2509–2520 (2015).
Clarke, N. et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 19, 975–986 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04030559 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04565496 (2020).
Tucker, M. D. et al. Pembrolizumab in men with heavily treated metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 37, 172–172 (2019).
Antonarakis, E. S. et al. Clinical features and therapeutic outcomes in men with advanced prostate cancer and DNA mismatch repair gene mutations. Eur. Urol. 75, 378–382 (2019).
Graff, J. N. et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget 7, 52810–52817 (2016).
Abida, W. et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 5, 471–478 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04737109 (2021).
De Bono, J. S. et al. Randomized phase II study evaluating AKT blockade with ipatasertib, in combination with abiraterone, in patients with metastatic prostate cancer with and without PTEN loss. Clin. Cancer Res. 25, 928–936 (2019).
Wang, Z. et al. The diverse roles of SPOP in prostate cancer and kidney cancer. Nat. Rev. Urol. 17, 339–350 (2020).
Boysen, G. et al. SpoP-mutated/CHD1-deleted lethal prostate cancer and abiraterone sensitivity. Clin. Cancer Res. 24, 5585–5593 (2018).
Blattner, M. et al. SPOP mutations in prostate cancer across demographically diverse patient cohorts. Neoplasia 16, 14–20 (2014).
McKay, R. R. et al. Results of a phase II trial of intense androgen deprivation therapy prior to radical prostatectomy (RP) in men with high-risk localized prostate cancer (PC). J. Clin. Oncol. 38, 5503–5503 (2020).
Shi, Q. et al. Prostate cancer-associated SPOP mutations enhance cancer cell survival and docetaxel resistance by upregulating Caprin1-dependent stress granule assembly. Mol. Cancer 18, 170 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04812366 (2021).
Acknowledgements
G.D. is a PhD fellow of the Research Foundation Flanders (FWO). W.D. is a recipient of the Emmanuel van der Schueren scholarship of “Kom Op Tegen Kanker”. W.E. is a Senior Clinical Investigator of the FWO. S.J. is a Senior Clinical Investigator of the FWO. This work was supported by the ‘Jozef De Wever Fonds voor prostaatkankerpreventie’ of the KU Leuven.
Author information
Authors and Affiliations
Contributions
G.D., W.D., F.C. and S.J. researched data for the article. G.D., W.D., G.D.M., M.B., F.C. and S.J. made a substantial contribution to discussion of content. G.D., W.D., M.B., F.C. and S.J. wrote the article. G.D., W.D., G.D.M., T.G., L.M., D.M., H.V.P., C.B., W.E., F.C. and S.J. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Urology thanks W. Dahut, E. Klein and J. Parsons for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
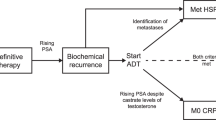
- Biochemical recurrence
-
(BCR). Rising PSA following treatment with curative intent (surgery or radiotherapy) for localized prostate cancer. Often defined as a (confirmed) rise in PSA to 0.2 ng/ml or more following radical prostatectomy, or a rise of 2 ng/ml or more above the PSA nadir after radiation therapy for localized prostate cancer.
- Hotspot mutations
-
Gain-of-function mutations.
- Poly(ADP-ribose) polymerase (PARP) inhibitor
-
A family of nuclear proteins involved in the single-strand break DNA repair pathway. Inhibition of poly(ADP-ribose) polymerase (PARP) is effective in men with mutations of the homologous recombination DNA repair pathway (such as BRCA1 and BRCA2), which promotes DNA double-strand break repair. PARP inhibition blocks single-strand break repair that evolves in a double-strand break, which cannot be repaired because of a deficiency of the homologous recombination DNA repair pathway.
Rights and permissions
About this article
Cite this article
Devos, G., Devlies, W., De Meerleer, G. et al. Neoadjuvant hormonal therapy before radical prostatectomy in high-risk prostate cancer. Nat Rev Urol 18, 739–762 (2021). https://doi.org/10.1038/s41585-021-00514-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-021-00514-9
This article is cited by
-
An MRI-based grading system for preoperative risk estimation of positive surgical margin after radical prostatectomy
Insights into Imaging (2023)
-
Recent advances and future perspectives in the therapeutics of prostate cancer
Experimental Hematology & Oncology (2023)
-
Neoadjuvant radiohormonal therapy for oligo-metastatic prostate cancer: safety and efficacy outcomes from an open-label, dose-escalation, single-center, phase I/II clinical trial
Frontiers of Medicine (2023)
-
Nomogram for predicting the biochemical recurrence of prostate cancer after neoadjuvant androgen deprivation therapy
International Urology and Nephrology (2023)
-
Association between age and efficacy of combination systemic therapies in patients with metastatic hormone-sensitive prostate cancer: a systematic review and meta-analysis
Prostate Cancer and Prostatic Diseases (2023)