Abstract
The term host defense peptides arose at the beginning to refer to those peptides that are part of the host’s immunity. Because of their broad antimicrobial capacity and immunomodulatory activity, nowadays, they emerge as a hope to combat resistant multi-drug microorganisms and emerging viruses, such as the case of coronaviruses. Since the beginning of this century, coronaviruses have been part of different outbreaks and a pandemic, and they will be surely part of the next pandemics, this review analyses whether these peptides and their derivatives are ready to be part of the treatment of the next coronavirus pandemic.
Similar content being viewed by others
Introduction
In the last few decades, there have been three important outbreaks related to coronaviruses; however, only the last one became pandemic. The first two coronavirus diseases: severe acute respiratory syndrome (SARS-CoV, 2002–2003) and the Middle East respiratory syndrome (MERS-CoV, 2012), showed less capacity to transmit from human-to-human thus the spreading worldwide was substantially minor compared with the novel coronavirus, which has extended worldwide leading to the considerable number of deaths (Parthasarathy and Vivekanandan 2020). Like the other two members, this new coronavirus belongs to the β-coronavirus genus and affects the lower respiratory tract and causes severe respiratory disorders and pneumonia in humans.
New promising drugs are starting to be used for the treatment of coronavirus-related diseases, nonetheless, none of them has shown complete clinical efficiency (Brodin 2021). Nowadays, several vaccines are being applied, mainly to the population that is considered within the higher-risk group. The vaccine application brings a new hope to stop this pandemic, but the creation and clinical studies of the different vaccines delayed a year, thus is necessary to have newly available treatments for future coronavirus pandemics. Currently, there is no-specific antiviral treatment available, indeed the treatment is focused on symptomatology and oxygen therapy, which represents the foremost intervention for patients with severe infection (Mohamed Khosroshahi et al. 2021). Thus, it is necessary to keep antiviral research on coronaviruses, immunomodulators and, drug repositioning for further treatment alternatives.
During coronavirus exposure, most individuals have an effective initial immune response, which eliminates the virus or leads to a subclinical infection with no symptoms or mild symptoms. In a few cases, viral evasion of the immune response can lead to refractory alveolar damage, ineffective lung repair mechanisms, and systemic hyper-inflammation with associated organ dysfunction worsening the patient’s outcome (Zheng et al. 2020). The immune response in these patients is highly variable and can include moderate to severe systemic inflammation or marked systemic immune suppression. Lately, several studies have suggested that an immunophenotype-driven approach such as anti-cytokine therapy or switching the proinflammatory stage to an immunoregulatory phenotype would help to succeed in treating patients with critical illness due to COVID-19. Although blocking antibodies therapy has been clinically tested and has shown promising results, the use of immunomodulators could improve the outcome.
The rationale of using a molecule that on one hand could lead to direct viral elimination and on the other hand promotes an anti-inflammatory profile puts in the spotlight some host defense peptides (HDPs) and their mimetic synthetic compounds, but do we have enough scientific research to propose these molecules as a new hope for future pandemics? In this review, we analyzed the potential use of HDPs and their synthetic counterparts as a further option for the treatment of coronavirus.
Coronavirus-Related Pandemics
In the middle of the 1960s, different laboratories isolated from the human respiratory tract a new virus with unusual properties (Tyrrell and Bynoe 1966). Later in the same decade, other researchers found a similar virus in animals: mice and swine. In the late 1960s, researchers reported that all these viruses were morphologically similar, this new group was named coronavirus, because of its crown-like appearance given by the spike protein (Kahn and McIntosh 2005). Currently, it is well known that Coronaviruses belong to the family Coronaviridae and subfamily Orthocoronavirinae. These viruses are RNA positive stranded, polyadenylated, infective, and can replicate in the cellular cytoplasm. This family has a unique characteristic to own the biggest genome reported among the enveloped RNA viruses, 27–32 kb, and is neatly packed along with the nucleocapsid protein (Brian and Baric 2005). All the coronaviruses share a similar structure in at least four important proteins: the spike glycoprotein (S protein), which constitutes the surface projections, a small envelope protein (E protein), a membrane glycoprotein (M protein), and a nucleocapsid protein (N protein) (Kahn and McIntosh 2005). Further, the coronaviruses are classified into α, β, γ, and δ genera; however, only the α and β viruses infect humans (Zheng et al. 2020).
Coronaviruses have been related to animals rather than humans, indeed, only seven sorts of coronaviruses can infect humans: HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1 are viruses that cause mild respiratory symptoms, whereas the pandemic viruses SARS-CoV, MERS-CoV, and SARS-CoV-2 cause severe acute respiratory symptoms. Bats and rodents are the main β-coronaviruses sources; however, there are other potential intermediate hosts, which finally can be the transmission source to humans (Zheng et al. 2020).
The first outbreak emerged in southern China and it was nominated as Severe Acute Respiratory Syndrome (SARS), and the etiological agent was recognized as SARS-CoV. During November 2002 and July 2003 around 8,000 cases of SARS were reported in 29 countries in North America, South America, Europe, and Asia, and 774 died, representing a mortality rate of 10% according to the Centers for Disease Control and Prevention (CDC 2003). Subsequent studies revealed that the SARS-CoV spread from bats to palm civets and subsequently to humans, probably originated through the selection and mutation of the SARS-like animal virus, which finally allowed human-to-human transmission (Halaji et al. 2020). Ten years later, in September 2012 arose the Middle East Respiratory Syndrome Coronavirus named MERS-CoV. The first report was in Saudi Arabia and all cases were established in the Middle East and North Africa region, Europe, East Asia, and the United States (Ramadan and Shaib 2019). The origin of this virus is not fully understood, but the evidence suggests bats as the primary host and then subsequent transmission to camels and humans. In 2012, MERS-CoV infected nearly 900 persons with 334 deaths resulting in a mortality rate of 35% (Halaji et al. 2020). To date, the World Health Organization (WHO) has notified 2,279 cases and 732 fatalities in 27 countries, thus MERS-CoV is by far the less contagious coronavirus, but it is the most fatal (Ramadan and Shaib 2019). The current pandemic is caused by a new emerging virus denominated Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) for its high similarity to SARS-CoV. This outbreak started in early December 2019 in Wuhan, China. In January 2020, the WHO announced a Public Health Emergency of International Concern, and finally, in March 2020 the WHO declared the disease COVID-19 as a pandemic. One year after, there are more than 100 million cases and more than 2 million deaths in at least 216 countries (Ganesh et al. 2021).
The MERS-CoV and SARS-CoV receptors are dipeptidyl peptidase 4 (DPP4) and angiotensin-converting enzyme 2 (ACE-2), respectively. These receptors are highly expressed in the respiratory tract mainly in epithelial cells. Both SARS viruses use the same mechanism for entry into host cells, but SARS-CoV-2 has around 10–20 times increased affinity and, therefore, higher infectivity rates. In the three infections, the symptoms are similar, including fever, myalgia, diarrhea, cough, and shortness of breath, with pneumonia or severe acute respiratory distress syndrome as the main complications (Ganesh et al. 2021). Besides, DPP4 is also expressed in kidneys, explaining common kidney failure in patients with MERS (Ramadan and Shaib 2019). Regarding the transmission route in coronavirus infections, the main lane in human-to-human is through the contact with oral, nasal, and eye mucous but also by coughing, sneezing, and inhaling respiratory droplets that contain the virus (Halaji et al. 2020).
No vaccination or convincing evidence for an effective treatment exists to MERS-CoV (Ramadan and Shaib 2019) and SARS-CoV (Stockman et al. 2006); however, in the absence of antiviral therapies, infected patients receive supporting therapy to avoid a critical state (Ganesh et al. 2021; Stockman et al. 2006). In contrast, in the current pandemic, there are essential advances regarding the development of efficient treatment or vaccines. Worthy to consider that RNA viruses have a high rate of genetic mutations that lead to the potential evolution of new resistant viral strains and, therefore, the possibility to evade the host immune response or avoid antiviral drugs (Brian and Baric 2005). Thus, it is important to consider new treatments to prevent future coronaviruses outbreaks.
Available Treatments and Vaccines, Is There Something Left?
Since the Sars-CoV-2-associated-disease, COVID-19, was officially known as the new pandemic, several clinical research groups started to seek potential treatments for novel coronavirus. Even though the scenery was not good, there was a history of previous coronavirus outbreaks, which allowed us a start, not from zero. Similar drugs that were used in previous pandemics began to be used and special attention was paid to the spike protein as a therapeutic target, in addition to using the artillery used to treat other types of RNA viruses (Khan et al. 2020). Several studies have suggested the use of interferon (IFN)-α, ribavirin combined with IFN-α or lopinavir/ritonavir, chloroquine phosphate, nelfinavir, and arbidol (Wolfel et al. 2020). Other antiviral drugs have been tested during this pandemic (reviewed comprehensively elsewhere) (Peng et al. 2021; Ratre et al. 2021), as well as the use of immunotherapy such as convalescent plasma from recovered patients, intravenous injection of an anti-SARS-CoV-2 human immunoglobulin, and tocilizumab, all of them accompanied by the use of glucocorticoids such as methylprednisolone to damper inflammation, thus allowing better oxygenation. Preventing complications and providing organ function support are also key therapeutic approaches; nevertheless, no specific antiviral treatment of COVID-19 still exists.
In the last months, several vaccines have emerged against the COVID-19, these vaccines came through because of the general global effort, a few candidates are in phase three and they are being used for the vaccination of susceptible groups, all of them with promissory results, many other candidates are in developing phases (WHO 2021). The development of these vaccines took about a year and during this time, clinical practitioners have been struggling with the COVID-19, using the drugs they have in their hands to save as many lives as they can. Therefore, we must have new alternatives for the next pandemic, which will happen undoubtedly, the question is when. There is no doubt that in that scenario, we will have the experience from this pandemic and some drugs ready to be used, of course, a new vaccine will be developed, but in the meantime, it will be necessary to provide pharmaceutical alternatives.
Role of HDPs to Control Viral Infections
Several HDPs have shown important antiviral activity against a wide variety of viruses (Ahmed et al. 2019). These peptides are fundamental components of the innate immune system and their role in viral infections has been highlighted in the past decades. HDPs are amphipathic small peptides with a net positive charge and are classified based on their structure as linear α-helical, β-sheet, cyclic peptides, and with extended flexible loop structures (Mookherjee et al. 2020). They can influence and modulate the immune response, beyond possess a wide microbicidal activity against bacteria, fungi, and viruses (Xhindoli et al. 2016). HDPs are synthesized and stored in immune and non-immune cells; they are expressed in a wide variety of tissues, mainly at sites normally exposed to higher loads of microbes such as the respiratory epithelium (Mookherjee et al. 2020).
HDP’s antiviral activity along with the IFN production help directly and indirectly in respiratory viral infections produced by enveloped virus mainly by the destabilization of the viral envelope, virion damage, and opsonization of viral particles, thus inhibiting the entry to host cells (Ahmed et al. 2019; Jenssen et al. 2006). To a lesser extent, HDPs have antiviral activity against non-enveloped viruses by decreasing viral replication or preventing nuclear entry of the viral genome (deeper analyzed in Wilson et al. 2013). Furthermore, HDPs promote an antiviral state by their immunomodulatory effects, including the enhancement of phagocyte function and orchestration of cytokine production (Mookherjee et al. 2020).
Human rhinoviruses (HRV) are the most common cause of viral respiratory infections in young people, the elderly, immunocompromised individuals, and patients with pre-existing pulmonary conditions. Some authors have reported that human cathelicidin LL-37 decreases viral HRV replication through a cytotoxic effect on the virions (Schögler et al. 2016) rather than in infected cells; however, LL-37 treatment also reduces the metabolism of the infected cells to avoid viral spreading (Sousa et al. 2017). Besides, the LL-37 absence during HRVs infections could lead to chronic obstructive pulmonary disease exacerbation (Mallia et al. 2012). Similarly, the enteroviruses elicit human β-defensin-3 (HBD-3) expression that can exert virucide extracellular activity (Chen et al. 2018) although this mechanism needs further examination.
Influenza A viruses (IAV) remain a major threat to human health because of past epidemics (Salvatore et al. 2007). Several mechanisms are related to the response of HDPs during IAV infection. For instance, the human neutrophil peptide (HNP)-1, which belongs to the α-defensins family, has shown the ability to inactivate IAV through direct binding to the virus, giving place to the formation of a complex, therefore preventing the entry of the virus into host cells and promoting phagocytosis (Daher et al. 1986). But also, there is evidence that HNP-1 inhibits protein kinase C activation in infected cells, which is required for viral replication and viral protein synthesis (Salvatore et al. 2007). Besides, HNPs were described as IAV viral particle aggregation inducers, facilitating their uptake by neutrophils (Tecle et al. 2007). Regarding human defensins (HD)-5 and HD-6, they both have similar activity as shown by HNP-1 against IAV but with lesser affinity (Doss et al. 2009). The IAV infection in plasmacytoid dendritic cells (DCs) and monocytes induced an early expression of human β-defensin (HBD)-1 and decreased viral replication, which suggests HBD-1 plays a protective role (Ryan et al. 2011). Besides, HBD-3 prevents the IAV fusion with the host cells by binding the viral glycoprotein hemagglutinin in a dose-dependent manner (Leikina et al. 2005). Complementary to α- and β-defensins, LL-37 has similar inhibition activity as HNPs, in this case, LL-37 damages viral IAV membranes and reduced the viral M protein generation in infected cells (Tripathi et al. 2013). Further, the LL-37 immunomodulatory properties are related to lesser disease severity since LL-37 treatment induces less secretion of proinflammatory cytokines in the lung (Barlow et al. 2011).
Human adenoviruses (HAdVs) are non-enveloped DNA viruses capable of infecting several systems, including the respiratory system, and frequently caused severe fatal disseminated infections in immunocompromised patients (Smith and Nemerow 2008). Both HNP-1 and HBD-2 are released by polymorphonuclear cells, reducing adenoviral infection in a dose-dependent manner (Bastian and Schafer 2001). Likewise, HD-5 and HBD-1 protect host cells from adenoviral infections (Gropp et al. 1999). These peptides bind to the viral capsid thus preventing viral uncoating and disabling it to penetrate cellular endosomes, which in turn hamper the adenovirus particles to spread, triggering a virion accumulation in early endosomes and lysosomes instead of their traffic to the nucleus (Nguyen et al. 2010; Smith and Nemerow 2008). To note, the correct peptide-folding and its net charge allow viral neutralization by binding to critical HAdV sites (Gounder et al. 2012; Smith et al. 2010). Less information exists regarding LL-37 effectiveness in HAdV infections. LL-37 exerts a rapid viral inactivation and the mechanism proposed is through the adenoviral particle disruption (Gordon et al. 2005). Nevertheless, this effect has only been observed in HAdV, probably because of their structural viral differences (Uchio et al. 2013).
The coronavirus outbreaks in the past years revealed the need for a deeper understanding of the immune processes to stop virus spreading, this knowledge will help us to develop new efficient therapies. The mechanisms described herein expose both, the redundancy and diversity in the functions of HDPs to neutralize viruses, placing these peptides as potential therapeutic molecules. Beyond the virucidal effects, HDPs also exert a wide immunomodulatory capacity that could lead to infection resolution (Fig. 1, Table 1).
Host defence peptides antiviral activity. (1) Host defence peptides (HDPs) have shown antiviral activity against the main viruses involved in respiratory infections such as adenoviruses (HAdV), rhinoviruses (HRVs), and Influenza A viruses (IAV). (2) HAdV bind the coxsackievirus and adenovirus receptor (CAR), HRVs bind to several glycoproteins such as ICAM-1 while IAV bind to hemagglutinin (HA) protein. (3) Once the viruses infect host cells, the expression of viral particles and specific HDPs is induced. (4) HAdV, HRVs, and IAV promote LL-37 expression; moreover, HRVs also induce human beta-defensin (HBD)-3, and IAV induce HBD-1 and human neutrophil peptide (HNP)-1 as well. (5) Some HDPs have direct virucidal effects. LL-37 can damage HAdV, HRVs, and IAV virions. Also, HBD-3 shows cytotoxic activity against HRVs. (6) Other HDPs have virucidal indirect effects. HNP-1 binds to HA protein, avoiding the IAV infection, but it also binds directly to the virus promoting its aggregation and inactivation. Besides HNP-1 inhibits protein C kinase (PKC) in infected cells, which is necessary for viral replication. To note, HD-5 and -6 have similar effects but with lesser potency. (7) In infected cells, HD-5 and HBD-2 bind directly to HAdV preventing its uncoating and promoting its accumulation in endosomes to avoid viral replication
What Do We Know About Coronavirus and HDPs?
To date, scarce information has been generated regarding the role of HDPs in response to the coronavirus infection, indeed, it is unknown whether the efficient production of these HDPs leads to the elimination of the coronavirus, causing mild or no symptomatology at all or if the low production or lack of HDPs could be associated with severe symptoms. The results generated from several studies in this context allow us to propose a new hypothesis of the possible role of these peptides. For instance, using molecular dynamic simulation, researchers determined that α-defensin-5 can block ACE-2 in the human intestinal epithelium, which was reflected in inhibiting the entry of SARS-CoV-2 pseudovirions to cells at concentrations as low as 10 μg/mL (Wang et al. 2020). Similarly, previous studies have shown that α defensins play an important role in preventing viral entry into host cells and in the transmission of viral infection among cells (Wilson et al. 2017). Previous studies reported that α-defensin levels were elevated in patients with COVID‐19, suggesting this defensin as a therapeutic target (Kerget et al. 2021). Other studies have demonstrated that β-defensins-derivative peptides exhibited potent and broad-spectrum antiviral effects on multiple respiratory viruses such as influenza A virus H1N1, H3N2, H5N1, H7N7, H7N9, SARS-CoV, and MERS-CoV. This antiviral activity was related to their high affinity to viral glycoproteins; they also prevent endosomal acidification by blocking membrane fusion and subsequent viral RNA release (Zhao et al. 2016). Similar studies in MERS-CoV infection showed that the conjugation of HBD-2 to spike protein receptor-binding domain induces antigen-specific protective immunity, thus suggesting that HBD-2 potentiates immune responses against the viral antigen and could be used as an adjuvant to enhance the immunogenicity of subunit vaccine candidates against MERS-CoV (Kim et al. 2020). Other approaches have emphasized the importance of HDPs to promote antiviral-related-immunity limiting viral proliferation in SARS-CoV-infected mice. The experimental use of defensins displayed significant reductions in levels of RANTES, IL-1α, IL-1β, IL-6, keratinocyte chemoattractant, MIP1α, monocyte chemoattractant protein 1, and IL-12 (p40), whereas the viral-control-related cytokines were increased (Wohlford-Lenane et al. 2009). Similar studies have reported that HBD-2 conjugated with the receptor-binding domain (RBD) of the S protein (S-RBD) present in MERS-CoV activates the primary antiviral innate response in monocytic cells, enhancing the expression of antiviral molecules such as type I IFN (IFN-I) and chemokines. Then, chemokines promote leukocyte recruitment, suggesting the induction of an effective adaptive response (Kim et al. 2018). Hence, S-RBD–HBD-2 promotes the expression of Nod2 and IFN-I through the activation of the transcription factor IRF3 to set up an antiviral cell state. Besides, HBD-2 induces the expression of M1 macrophage markers which are associated with antigenic presentation and antibody production (Kim et al. 2019). Thus, immunization with S-RBD–HBD-2 leads to a protective immune response against MERS-CoV and could be used as an approach for vaccine development (Kim et al. 2018, 2019, 2020).
Moreover, the HD-5 structure allows interaction with the SARS-CoV-2 receptor, ACE-2, even with a higher affinity than the virus itself. HD-5 hides amino acids crucial for the ACE-2 recognition by the S protein and hence, HD-5 competes with the virus inhibiting its entry to host cells (Wang et al. 2020). These results agree with a study where patients with COVID-19 showed reduced expression levels of defensins, such as HBD4A, HBD106B, HBD107B, HBD103A, and HD1B in the nasopharyngeal cavity (Idris et al. 2020). Thus, suggesting that insufficient production of defensins allows progressive disease and it is also proposed the use of an HD-5 aerosol before potential exposure to SARS-CoV-2 as a preventive measure (Niv 2020).
Less information is available regarding other HDPs such as LL-37 or the antimicrobial protein lactoferrin (Salvatore et al. 2007). SARS-CoV promotes high lactoferrin expression in polymorphonuclear cells (Reghunathan et al. 2005), which in turn inhibits viral entry into host cells since both lactoferrin and SARS-CoV compete for the same receptor (Elnagdy and AlKhazindar 2020; Lang et al. 2011). Similar results have been proposed for SARS-CoV-2 because of the similitude between both viruses.
Recent studies have demonstrated that LL-37 binds to SARS-CoV-2 S protein and inhibits binding to its receptor hACE2, and most likely viral entry into the cell, in vitro. Several reports suggest that vitamin D supplementation is associated with a lower risk of SARS-CoV-2 infection or disease progression (Entrenas-Castillo et al. 2020; Grant et al. 2020a, 2020b; Mercola et al. 2020; Panfili et al. 2021). This protective effect could be mediated by LL-37 immunomodulatory properties since vitamin D is the main LL-37 inducer through the activation of the vitamin D response elements located in the promoter of the hCAP18 gene, which is the precursor of LL-37 mature peptide (Beard et al. 2011).
Other approaches have been suggested to counterattack coronavirus infection, for instance, the peptide OC43-HR2P, derived from the heptad repeat 2 (HR2) domain of HCoV-OC43, exhibited a broad fusion inhibitory activity against multiple HCoVs. EK1, the optimized form of OC43-HR2P, showed substantially improved pan-CoV fusion inhibitory activity, indeed, lipopeptides derived from EK1 showed important fusion inhibitor activity against SARS-CoV-2 S protein-mediated membrane fusion (Xia et al. 2019, 2020). Thus, pan-coronavirus fusion inhibitory peptides are potential candidates for pharmaceutical development.
HDPs Immunomodulatory Capacity Could be Used for Treating Coronavirus
Although the proinflammatory activity of HDPs has been widely described in the past decade, several findings have demonstrated that HDPs under certain conditions selectively act as anti-inflammatory molecules. Indeed, some HDPs are balanced toward anti-inflammatory activity, such as the case for LL-37. Administration of exogenous LL-37 decreased tumor necrosis factor (TNF)-α) and interleukin (IL)-17 while inducing anti-inflammatory IL-10 and transforming growth factor (TGF)-β production in Mycobacterium tuberculosis-infected macrophages, whereas in uninfected macrophages promoted a proinflammatory profile (Torres-Juarez et al. 2015). This cathelicidin is also capable of inhibiting neutrophil chemotaxis, inducing the internalization of the CXCR2 (Zhang et al. 2009) and promoting non-inflammatory necrosis, thus controlling excessive inflammation (Li et al. 2009). Another anti-inflammatory mechanism described for LL-37 is that this peptide modulates the TLR-to-NFκB pathway contributing to the local regulation of inflammation, furthermore, LL-37 promotes the production of IL-1-soluble receptor, whereas induces the expression of anti-inflammatory cytokines such as IL-10 and TGF-β (Hemshekhar et al. 2018; Mookherjee et al. 2006, 2009; Torres-Juarez et al. 2015). Interestingly, other molecular mechanisms are involved with the capacity of LL-37 to inhibit the Th1 immune responses produced in response to IFN-γ by suppressing the production of TNF-α and IL-12 in monocytes, macrophages, and DCs, as well as inhibiting the activation of class-switching in splenic B cells, these inhibitory effects are merely mediated through suppression of STAT1-independent-signaling pathway (Nijnik et al. 2009). KR-12 is a short fragment from LL-37 corresponding to residues 18–29 and exhibits antimicrobial activity and important anti-inflammatory activity, indeed, alleviates inflammation in colitis models, this suggests that KR-12 is worth being considered as a potential therapeutic, nonetheless, the mechanism to reduce inflammation has not been elucidated (Fabisiak et al. 2021). Similar studies have reported that LL-37 restored glucocorticoid sensitivity impaired by dsRNA, possibly by inhibiting the Akt pathway, in addition to Erk1/2 pathway. These findings suggest LL-37 as a therapeutic agent for treating viral infections in inflammatory pulmonary diseases (Li et al. 2020).
While LL-37 and its derivatives have shown clear evidence to modulate the immune response, other HDPs have similar functions and can even act synergically. The HBD-3 has recently been associated with anti-inflammation, this defensin can attenuate the production of IL-6, IL-10, GM-CSF, and TNF-α response of human myeloid DCs (Pingel et al. 2008). Other studies showed that in tuberculosis patients the concentration of cortisol correlates positively with the levels of HBD-3, which strongly suggests its relation in anti-inflammatory processes (Bongiovanni et al. 2020). In the same line, HBD-3 can polarize macrophages into M2 phenotype contributing to an anti-inflammatory environment (Lyu et al. 2017). HBD-3 also is a potent inhibitor of the accumulation of proinflammatory cytokines such as TNF-α and IL-6, both in vivo and in vitro; overall, these studies suggest a role of HBD-3 in the resolution of inflammation, which is necessary to avoid tissue damage by effectors of antimicrobial action and cytokine storm (Semple et al. 2010). On the other hand, α-defensin (HNP-1) blocked the release of IL-1β from LPS-activated monocytes, but not the expression and release of TNF-α (Shi et al. 2007).
In severe COVID-19, there is an exacerbated immune response that promotes important tissue damage. Considering that the main HDPs antimicrobial mechanisms are through the lysis of pathogenic membranes and immunoregulation, these peptides can be selectively induced, thus promoting anti-inflammatory activity while eliminating viral particles, thus avoiding tissue damage (Garcia-Fandino and Pineiro 2021). Additionally, the α-defensins released by apoptotic neutrophils inhibit the biosynthesis of pro-inflammatory cytokines by macrophages (Brook et al. 2016). In summary, this evidence suggests that HDPs' cooperative action could reduce or control the tissue damage induced by excessive inflammation during viral infections, such as in COVID-19.
What Do We Have: Clinical Trials with Antiviral HDP, Peptoids, and Synthetic Peptides
The emerging novel viruses have prompted the research for new antiviral therapies, putting into the spotlight HDPs because of their antiviral and immunomodulatory features. Although thousands of HDPs have been reported, only a few of them have reached clinical trials, and even fewer have been approved for clinical practice.
Because of the widely documented antiviral activity of HDPs, several groups have explored other related options such as peptoids, which are peptidomimetics described as foldamers consisting of N-alkylated glycine oligomers; lactoferrin, which is an antimicrobial protein with known antiviral activity for viruses based on DNA and RNA; and synthetic peptides, which have proven an excellent type of molecule for the mimicry of protein/peptides activity, most of them have diverse chemical modifications, which includes the incorporation of a large range of non-proteinogenic amino acids and the modification of the peptide backbone. Apart from extending the chemical and structural diversity presented by peptides, such modifications also increase the proteolytic stability of the molecules, and the affinity for certain binding sites, enhancing their utility for clinical applications.
The most representative peptide for clinical use in viral infections is Enfuvirtide (T20), which is approved in patients with HIV (Poveda et al. 2005). This peptide has 36 amino acids with a conserved region called HR2 (C-terminal heptad repeat), which disrupts the HIV-1 molecular machinery at the final stage of fusion with the target cell, preventing uninfected cells from becoming infected. Enfuvirtide was designed to mimic components of the HIV-1 fusion machinery and displace them, is often used for treating patients that do not respond to retroviral therapy (Poveda et al. 2005). Sifuvirtide has 36 amino acids in length and shares some sequence and structure properties with the native CHR peptide of HIV-1 gp41 glycoprotein. In comparison with T-20, it has 22 different amino acids residues. It has an increased half-life than T-20 and is highly effective against T-20-resistant strains. The use of sifuvirtide is still in phase III clinical trials in China (He et al. 2008), interestingly, sifuvirtide-resistant strains have already been characterized and mechanisms underlying this resistance have been described (Yu et al. 2018).
The synthetic peptide derivatives not only have been used to control HIV infection but also in opportunistic viral infections. Synthetic Ezrin Peptide One (HEP1) therapy reduced the incidence of opportunistic infections. The result of clinical trial phases I and II reported that the incidence of viral infection decreased for herpes zoster, vaginal candidiasis, oral candidiasis, and acne vulgaris (Salamov et al. 2007). Another mechanism to alter or enhance the properties of the peptide is the use of a combination of drugs. The clinical phase III trial of Boceprevir (a linear peptidomimetic NS3/4A serine protease inhibitor), with peginterferon/ribavirin, shows that the combination was effective in adults with chronic hepatitis C who failed response with prior peginterferon/ribavirin treatment. The mechanism of action is a NS3/4a protease inhibitor used to inhibit viral replication (Vierling et al. 2014). These drugs are evidence for the use of synthetic peptides that provide a predictive mechanism of action. Similarly, brilacidin is peptidomimetic, with non-peptidic scaffolds and side chains, which has structural and biological properties similar to HDPs, it has been tested in phase II for skin acute bacterial infection, ulcerative proctitis, and viral infections. Recently, this peptidomimetic has been evaluated in a human lung cell line and Vero cells. These results suggest that SARS-CoV-2 inhibition in these cell culture models is likely to be a result of the impact of brilacidin on viral entry and its disruption of viral integrity (Bakovic et al. 2021).
The polypeptide aprotinin from bovine lung is a broad-range inhibitor of serine proteases that exhibits antimicrobial activity against different microorganisms. The safety and efficacy of aprotinin were shown in clinical trials (Schütz et al. 2020), and now the aprotinin has been experimentally used in Calu-3 human airway epithelial cells infected with SARS-CoV2 showing considerable therapeutic potential for the treatment of COVID-19 (Bestle et al. 2020). Indeed, aprotinin inhibited SARS-CoV-2 replication in therapeutically achievable concentrations. An analysis of proteomics and translatome data indicated that SARS-CoV-2 replication is associated with a downregulation of host cell protease inhibitors; thus, aprotinin may interfere with SARS-CoV-2-mediated downregulation of host cell protease inhibitors during later virus replication cycles (Bojkova et al. 2020). As an anti-influenzal compound, aprotinin used as small-particle aerosol has been approved in Russia for local respiratory application in mild-to-moderate influenza to provide both an antiviral effect and a decrease in systemic pathology and inflammation (Zhirnov et al. 2011). Regardless, the use of aprotinin in clinics for coronavirus infections deserves further investigation.
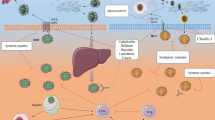
The most recent use of an antimicrobial protein from animals in a clinical trial is the treatment with lactoferrin (Chang et al. 2020). Lactoferrin is a glycoprotein found predominantly in milk, several studies have confirmed its antiviral activity against several viruses, including SARS-CoV-2, through the blocking of the viral receptors on the host cells thus preventing the entry and replication. Data revealed that lactoferrin interacts with heparan sulfate proteoglycans and ACE-2 receptors that are reported as SARS-CoV-2-binding sites to enter the host cell, suggesting a potential significance of lactoferrin as an antiviral against SARS-CoV-2 (Fig. 2). Moreover, the immunoregulatory effects of lactoferrin can protect against the cytokine storm and thrombotic complications that result from the COVID-19 infection (Chang et al. 2020) (Table 2).
Potential activity of HDPs during coronavirus infection. (1) SARS-CoV-2 spike protein binds to ACE-2 receptors expressed mainly in epithelial cells. (2) However, the presence of lactoferrin and HD-5 can block ACE-2 and inhibits the entry of SARS-CoV-2. (3) Once this virus infects the epithelial cells, the expression of HDPs is regulated. (4A) The genetic expression of LL-37 increases in infected cells and, LL-37 has antiviral direct activity; (4B) whereas the expression of defensins is downregulated; however, HBD-2 is a cytokine regulator
Scenarios that Should be Considered
Some authors have highlighted that HDPs are involved in the immunopathology of COVID-19, the mechanisms have not been deeply studied, however, the researchers hypothesize that the massive modification of the altered host membranes by the virus triggers the response of natural HDPs by destroying them as they do with the membranes of other pathogenic agents. In theory, this model could contribute explaining the first cause of death by COVID-19: acute respiratory failure due to the self-immune disruption of the lung cells; therefore, the reestablishment of lipid composition or even the blockage of specific HDPs involved in the destruction of host cells could be considered as possible therapeutic intervention point (Garcia-Fandino and Pineiro 2021). The design and use of HDPs and peptide-derived therapeutics must be focused on those with anti-inflammatory and specific anti-viral activity. Besides, it will be necessary to determine specifically what HDPs are involved in the immunopathogenesis of COVID-19 to specifically block those detrimental peptides.
Future Perspectives
As previously mentioned, HDPs have a wide variety of functions that can influence the onset of the immune system, on one hand, promoting inflammatory response and on the other hand promoting anti-inflammation (Rivas-Santiago and Torres-Juarez 2018), which not necessarily means abrogation of direct antiviral activity. Since the immunopathology of Coronavirus-related diseases is most inflammatory, the use of antimicrobial peptides and their derivatives as therapeutics must be deeply evaluated and research should be focused on those candidates with markedly anti-inflammatory effects and prominent antiviral activity, as described above. There are several candidates and many more will emerge using bioinformatic designing choosing or modeling the ideal HDPs. We should not forget the lessons learned from this pandemic and we should be prepared well in advance, planning appropriate preventive measures for the next wave of the outbreak of coronavirus in the future; thus, the question should not be whether or not there will be another pandemic, the correct question is when it will happen, by then, we should have a long list of candidates ready to be used.
References
Ahmed A, Siman-Tov G, Hall G et al (2019) Human antimicrobial peptides as therapeutics for viral infections. Viruses 11:704. https://doi.org/10.3390/v11080704
Bakovic A, Risner K, Bhalla N et al (2021) Brilacidin demonstrates inhibition of SARS-CoV-2 in cell culture. Viruses 13:271. https://doi.org/10.3390/v13020271
Barlow P, Svoboda P, Mackellar A et al (2011) Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS ONE 6:e25333. https://doi.org/10.1371/journal.pone.0025333
Bastian A, Schafer H (2001) Human alpha-defensin 1 (HNP-1) inhibits adenoviral infection in vitro. Regul Pept 101:157–161. https://doi.org/10.1016/s0167-0115(01)00282-8
Beard J, Bearden A, Striker R (2011) Vitamin D and the anti-viral state. J Clin Virol 50:194–200. https://doi.org/10.1016/j.jcv.2010.12.006
Bestle D, Heindl MR, Limburg H et al (2020) TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci Alliance 3:e202000786. https://doi.org/10.26508/lsa.202000786
Bojkova D, Bechtel M, McLaughlin KM et al (2020) Aprotinin inhibits SARS-CoV-2 replication. Cells 9:2377. https://doi.org/10.3390/cells9112377
Bongiovanni B, Marin-Luevano S, D’Attilio L et al (2020) Evidence that changes in antimicrobial peptides during tuberculosis are related to disease severity, clinical presentation, specific therapy and levels of immune-endocrine mediators. Cytokine 126:154913. https://doi.org/10.1016/j.cyto.2019.154913
Brian D, Baric R (2005) Coronavirus genome structure and replication. Curr Top Microbiol Immunol 287:1–30. https://doi.org/10.1007/3-540-26765-4_1
Brodin P (2021) Immune determinants of COVID-19 disease presentation and severity. Nat Med 27:28–33. https://doi.org/10.1038/s41591-020-01202-8
Brook M, Tomlinson GH, Miles K et al (2016) Neutrophil-derived alpha defensins control inflammation by inhibiting macrophage mRNA translation. Proc Natl Acad Sci USA 113:4350–4355. https://doi.org/10.1073/pnas.1601831113
CDC (2003) Revised U.S. surveillance case definition for severe acute respiratory syndrome (SARS) and update on SARS cases--United States and worldwide, December 2003. MMWR Morb Mortal Wkly Rep 52:1202–1206. https://pubmed.ncbi.nlm.nih.gov/14668711/
Chang R, Ng TB, Sun WZ (2020) Lactoferrin as potential preventative and adjunct treatment for COVID-19. Int J Antimicrob Agen 56:106118. https://doi.org/10.1016/j.ijantimicag.2020.106118
Chen W, Liu Z, Zhang Q et al (2018) Induction and antiviral activity of human β-defensin 3 in intestinal cells with picornavirus infection. Acta Virol 62:287–293. https://doi.org/10.4149/av_2018_222
Daher K, Selsted M, Lehrer R (1986) Direct inactivation of viruses by human granulocyte defensins. J Virol 60:1068–1074. https://doi.org/10.1128/JVI.60.3.1068-1074.1986
Doss M, White M, Tecle T et al (2009) Interactions of alpha-, beta-, and theta-defensins with influenza A virus and surfactant protein D. J Immunol 182:7878–7887. https://doi.org/10.4049/jimmunol.0804049
Elnagdy S, AlKhazindar M (2020) The potential of antimicrobial peptides as an antiviral therapy against COVID-19. ACS Pharmacol Transl Sci 3:780–782. https://doi.org/10.1021/acsptsci.0c00059
Entrenas-Castillo M, Entrenas-Costa LM, Vaquero-Barrios JM et al (2020) Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J Steroid Biochem Mol Biol 203:105751. https://doi.org/10.1016/j.jsbmb.2020.105751
Fabisiak N, Fabisiak A, Chmielowiec-Korzeniowska A et al (2021) Anti-inflammatory and antibacterial effects of human cathelicidin active fragment KR-12 in the mouse models of colitis: a novel potential therapy of inflammatory bowel diseases. Pharmacol Rep 73:163–171. https://doi.org/10.1007/s43440-020-00190-3
Ganesh B, Rajakumar T, Malathi M et al (2021) Epidemiology and pathobiology of SARS-CoV-2 (COVID-19) in comparison with SARS, MERS: An updated overview of current knowledge and future perspectives Clin Epidemiol Global Health 10:100694. https://doi.org/10.1016/j.cegh.2020.100694
Garcia-Fandino R, Pineiro A (2021) Delving into the origin of destructive inflammation in COVID-19: A betrayal of natural host defense peptides? Front Immunol 11:610024. https://doi.org/10.3389/fimmu.2020.610024
Gordon J, Huang L, Romanowski E et al (2005) Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr Eye Res 30:385–394. https://doi.org/10.1080/02713680590934111
Gounder A, Wiens M, Wilson S et al (2012) Critical determinants of human α-defensin 5 activity against non-enveloped viruses. J Biol Chem 287:24554–24562. https://doi.org/10.1074/jbc.M112.354068
Grant W, Lahore H, McDonnell S, S, et al (2020a) Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 12:988. https://doi.org/10.3390/nu12040988
Grant W, Lahore H, Rockwell M (2020b) The benefits of vitamin D supplementation for athletes: better performance and reduced risk of COVID-19. Nutrients 12:3741. https://doi.org/10.3390/nu12123741
Gropp R, Frye M, Wagner T et al (1999) Epithelial defensins impair adenoviral infection: implication for adenovirus-mediated gene therapy. Hum Gene Ther 10:957–964. https://doi.org/10.1089/10430349950018355
Halaji M, Farahani A, Ranjbar R et al (2020) Emerging coronaviruses: first SARS, second MERS and third SARS-CoV-2: epidemiological updates of COVID-19. Infez Med 28(suppl 1):6–17
He Y, Xiao Y, Song H et al (2008) Design and evaluation of sifuvirtide, a novel HIV-1 fusion inhibitor. J Biol Chem 283:11126–11134. https://doi.org/10.1074/jbc.M800200200
Hemshekhar M, Choi KG, Mookherjee N (2018) Host defense peptide LL-37-mediated chemoattractant properties, but not anti-inflammatory cytokine IL-1RA production, is selectively controlled by Cdc42 Rho GTPase via G protein-coupled receptors and JNK mitogen-activated protein kinase. Front Immunol 9:1871. https://doi.org/10.3389/fimmu.2018.01871
Idris M, Banu S, Siva A et al (2020) Downregulation of Defensin genes in SARS-CoV-2 infection. medRxiv https://doi.org/10.1101/2020.09.21.20195537
Jenssen H, Hamill P, Hancock R (2006) Peptide antimicrobial agents. Clin Microbiol Rev 19:491–511. https://doi.org/10.1128/CMR.00056-05
Kahn JS, McIntosh K (2005) History and recent advances in coronavirus discovery. Pediatr Infect Dis J 24:S223–S227. https://doi.org/10.1097/01.inf.0000188166.17324.60
Kerget B, Kerget F, Aksakal A et al (2021) Evaluation of alpha defensin, IL-1 receptor antagonist, and IL-18 levels in COVID-19 patients with macrophage activation syndrome and acute respiratory distress syndrome. J Med Virol 93:2090–2098. https://doi.org/10.1002/jmv.26589
Khan S, Siddique R, Shereen MA et al (2020) Emergence of a novel coronavirus, severe acute respiratory syndrome coronavirus 2: biology and therapeutic options. J Clin Microbiol 58:e00187-e220. https://doi.org/10.1128/JCM.00187-20
Kim J, Yang YL, Jang SH et al (2018) Human β-defensin 2 plays a regulatory role in innate antiviral immunity and is capable of potentiating the induction of antigenspecific immunity. Virol J 15:124. https://doi.org/10.1186/s12985-018-1035-2
Kim J, Yang YL, Jang YS (2019) Human β-defensin 2 is involved in CCR2-mediated Nod2 signal transduction, leading to activation of the innate immune response in macrophages. Immunobiology 224:502–510. https://doi.org/10.1016/j.imbio.2019.05.004
Kim J, Yang YL, Jeong Y et al (2020) Conjugation of human beta-defensin 2 to spike protein receptor-binding domain induces antigen-specific protective immunity against middle east respiratory syndrome coronavirus infection in human dipeptidyl peptidase 4 transgenic mice. Vaccines 8:635. https://doi.org/10.3390/vaccines8040635
Lang J, Yang N, Deng J et al (2011) Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PLoS ONE 6:e23710. https://doi.org/10.1371/journal.pone.0023710
Leikina E, Delanoe-Ayari H, Melikov K et al (2005) Carbohydrate-binding molecules inhibit viral fusion and entry by crosslinking membrane glycoproteins. Nat Immunol 6:995–1001. https://doi.org/10.1038/ni1248
Li HN, Barlow PG, Bylund J et al (2009) Secondary necrosis of apoptotic neutrophils induced by the human cathelicidin LL-37 is not proinflammatory to phagocytosing macrophages. J Leukoc Biol 86:891–902. https://doi.org/10.1189/jlb.0209050
Li K, Tao N, Zheng L et al (2020) LL-37 restored glucocorticoid sensitivity impaired by virus dsRNA in lung. Int Immunopharmacol 79:106057. https://doi.org/10.1016/j.intimp.2019.106057
Lyu J, Bian T, Chen B et al (2017) Beta-defensin 3 modulates macrophage activation and orientation during acute inflammatory response to Porphyromonas gingivalis lipopolysaccharide. Cytokine 92:48–54. https://doi.org/10.1016/j.cyto.2016.12.015
Mallia P, Footitt J, Sotero R et al (2012) Rhinovirus Infection induces degradation of antimicrobial peptides and secondary bacterial infection in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 186:1117–1124. https://doi.org/10.1164/rccm.201205-0806OC
Mercola J, Grant W, Wagner C (2020) Evidence regarding vitamin D and risk of COVID-19 and its severity. Nutrients 12:3361. https://doi.org/10.3390/nu12113361
Mohamed Khosroshahi L, Rokni M, Mokhtari T et al (2021) Immunology, immunopathogenesis and immunotherapeutics of COVID-19; an overview. Int Immunopharmacol 93:107364. https://doi.org/10.1016/j.intimp.2020.107364
Mookherjee N, Brown KL, Bowdish DM et al (2006) Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J Immunol 176:2455–2464. https://doi.org/10.4049/jimmunol.176.4.2455
Mookherjee N, Anderson M, Haagsman H et al (2020) Antimicrobial host defence peptides: functions and clinical potential. Nat Rev Drug Discov 19:311–332. https://doi.org/10.1038/s41573-019-0058-8
Mookherjee N, Hamill P, Gardy J et al (2009) Systems biology evaluation of immune responses induced by human host defence peptide LL-37 in mononuclear cells. Mol Biosyst 5:483–496. https://doi.org/10.1039/b813787k
Nguyen E, Nemerow G, Smith J (2010) Direct evidence from single-cell analysis that human -defensins block adenovirus uncoating to neutralize infection. J Virol 84:4041–4049. https://doi.org/10.1128/JVI.02471-09
Nijnik A, Pistolic J, Wyatt A et al (2009) Human cathelicidin peptide LL-37 modulates the effects of IFN-gamma on APCs. J Immunol 183:5788–5798. https://doi.org/10.4049/jimmunol.0901491
Niv Y (2020) Defensin 5 for prevention of SARS-CoV-2 invasion and Covid-19 disease. Med Hypotheses 143:110244. https://doi.org/10.1016/j.mehy.2020.110244
Panfili F, Roversi M, Argenio P et al (2021) Possible role of vitamin D in Covid-19 infection in pediatric population. J Endocrinol Invest 44:27–35. https://doi.org/10.1007/s40618-020-01327-0
Parthasarathy P, Vivekanandan S (2020) An extensive study on the COVID-19 pandemic, an emerging global crisis: Risks, transmission, impacts and mitigation. J Infect Public Health 14:249–259. https://doi.org/10.1016/j.jiph.2020.12.020
Peng Y, Tao H, Satyanarayanan SK et al (2021) A comprehensive summary of the knowledge on COVID-19 treatment. Aging Dis 12:155–191
Pingel LC, Kohlgraf KG, Hansen CJ et al (2008) Human beta-defensin 3 binds to hemagglutinin B (rHagB), a non-fimbrial adhesin from Porphyromonas gingivalis, and attenuates a pro-inflammatory cytokine response. Immunol Cell Biol 86:643–649. https://doi.org/10.1038/icb.2008.56
Poveda E, Briz V, Soriano V (2005) Enfuvirtide, the first fusion inhibitor to treat HIV infection. AIDS Rev 7:139–147
Ramadan N, Shaib H (2019) Middle East respiratory syndrome coronavirus (MERS-CoV): A review. Germs 9:35–42. https://doi.org/10.18683/germs.2019.1155
Ratre YK, Kahar N, Bhaskar L et al (2021) Molecular mechanism, diagnosis, and potential treatment for novel coronavirus (COVID-19): a current literature review and perspective. 3 Biotech 11:94. https://doi.org/10.1007/s13205-021-02657-3
Reghunathan R, Jayapal M, Hsu LY et al (2005) Expression profile of immune response genes in patients with Severe Acute Respiratory Syndrome. BMC Immunol 6:2. https://doi.org/10.1186/1471-2172-6-2
Rivas-Santiago B, Torres-Juarez F (2018) Antimicrobial peptides for the treatment of pulmonary tuberculosis, allies or foes? Curr Pharm Des 24:1138–1147. https://doi.org/10.2174/1381612824666180327162357
Ryan L, Dai J, Yin Z et al (2011) Modulation of human β-defensin-1 (hBD-1) in plasmacytoid dendritic cells (PDC), monocytes, and epithelial cells by influenza virus, Herpes simplex virus, and Sendai virus and its possible role in innate immunity. J Leukoc Biol 90:343–356. https://doi.org/10.1189/jlb.0209079
Salamov G, Holms R, Bessler WG et al (2007) Treatment of hepatitis C virus infection with human ezrin peptide one (HEP1) in HIV infected patients. Arzneimittelforschung 57:497–504. https://doi.org/10.1055/s-0031-1296637
Salvatore M, García-Sastre A, Ruchala P et al (2007) alpha-Defensin inhibits influenza virus replication by cell-mediated mechanism(s). J Infect Dis 196:835–843. https://doi.org/10.1086/521027
Schögler A, Muster R, Kieninger E et al (2016) Vitamin D represses rhinovirus replication in cystic fibrosis cells by inducing LL-37. Eur Respir J 47:520–530. https://doi.org/10.1183/13993003.00665-2015
Schütz D, Ruiz-Blanco Y, Münch J et al (2020) Peptide and peptide-based inhibitors of SARS-CoV-2 entry. Adv Drug Deliv Rev 167:47–65. https://doi.org/10.1016/j.addr.2020.11.007
Semple F, Webb S, Li HN et al (2010) Human beta-defensin 3 has immunosuppressive activity in vitro and in vivo. Eur J Immunol 40:1073–1078. https://doi.org/10.1002/eji.200940041
Shi J, Aono S, Lu W et al (2007) A novel role for defensins in intestinal homeostasis: regulation of IL-1beta secretion. J Immunol 179:1245–1253. https://doi.org/10.4049/jimmunol.179.2.1245
Smith J, Nemerow G (2008) Mechanism of Adenovirus neutralization by human a-defensins. Cell Host Microbe 3:11–19. https://doi.org/10.1016/j.chom.2007.12.001
Smith J, Silvestry M, Lindert S et al (2010) Insight into the mechanisms of adenovirus capsid disassembly from studies of defensin neutralization. PLoS Pathog 6:e1000959. https://doi.org/10.1371/journal.ppat.1000959
Sousa FH, Casanova V, Findlay F et al (2017) Cathelicidins display conserved direct antiviral activity towards rhinovirus. Peptides 95:76–83. https://doi.org/10.1016/j.peptides.2017.07.013
Stockman L, Bellamy R, Garner P (2006) SARS: systematic review of treatment effects. PLoS Med 3:e343. https://doi.org/10.1371/journal.pmed.0030343
Tecle T, White M, Gantz D et al (2007) Human neutrophil defensins increase neutrophil uptake of influenza A virus and bacteria and modify virus-induced respiratory burst responses. J Immunol 178:8046–8052. https://doi.org/10.4049/jimmunol.178.12.8046
Torres-Juarez F, Cardenas-Vargas A, Montoya-Rosales A et al (2015) LL-37 immunomodulatory activity during Mycobacterium tuberculosis infection in macrophages. Infect Immun 83:4495–4503. https://doi.org/10.1128/IAI.00936-15
Tripathi S, Tecle T, Verma A et al (2013) The human cathelicidin LL-37 inhibits influenza A viruses through a mechanism distinct from that of surfactant protein D or defensins. J General Virol 94(Pt 1):40–49. https://doi.org/10.1099/vir.0.045013-0
Tyrrell D, Bynoe M (1966) Cultivation of viruses from a high proportion of patients with colds. Lancet 1:76–77. https://doi.org/10.1016/s0140-6736(66)92364-6
Uchio E, Inoue H, Kadonosono K (2013) Anti-adenoviral effects of human cationic antimicrobial protein-18/LL-37, an Antimicrobial peptide, by quantitative polymerase chain reaction. Korean J Ophthalmol 27:199–203. https://doi.org/10.3341/kjo.2013.27.3.199
Vierling JM, Davis M, Flamm S et al (2014) Boceprevir for chronic HCV genotype 1 infection in patients with prior treatment failure to peginterferon/ribavirin, including prior null response. J Hepatol 60:748–756. https://doi.org/10.1016/j.jhep.2013.12.013
Wang C, Wang S, Li D et al (2020) Human Intestinal Defensin 5 Inhibits SARS-CoV-2 Invasion by Cloaking ACE2. Gastroenterology 159:1145-1147e1144. https://doi.org/10.1053/j.gastro.2020.05.015
WHO (2021) https://www.who.int/publications/m/item/draftlandscape-of-covid-19-candidate-vaccines
Wilson S, Wiens M, Smith J (2013) Antiviral mechanisms of human defensins. J Mol Biol 425:4965–4980. https://doi.org/10.1016/j.jmb.2013.09.038
Wilson SS, Bromme BA, Holly MK et al (2017) Alpha-defensin-dependent enhancement of enteric viral infection. PLoS Pathog 13:e1006446. https://doi.org/10.1371/journal.ppat.1006446
Wohlford-Lenane CL, Meyerholz DK, Perlman S et al (2009) Rhesus theta-defensin prevents death in a mouse model of severe acute respiratory syndrome coronavirus pulmonary disease. J Virol 83:11385–11390. https://doi.org/10.1128/JVI.01363-09
Wolfel R, Corman VM, Guggemos W et al (2020) Virological assessment of hospitalized patients with COVID-2019. Nature 581:465–469. https://doi.org/10.1038/s41586-020-2196-x
Xhindoli D, Pacor S, Benincasa M et al (2016) The human cathelicidin LL-37–A pore-forming antibacterial peptide and host-cell modulator. Biochim Biophys Acta 1858:546–566. https://doi.org/10.1016/j.bbamem.2015.11.003
Xia S, Liu M, Wang C et al (2020) Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res 30:343–355. https://doi.org/10.1038/s41422-020-0305-x
Xia S, Yan L, Xu W et al (2019) A pan-coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike. Sci Adv. https://doi.org/10.1126/sciadv.aav4580
Yu D, Ding X, Liu Z et al (2018) Molecular mechanism of HIV-1 resistance to sifuvirtide, a clinical trial-approved membrane fusion inhibitor. J Biol Chem 293:12703–12718. https://doi.org/10.1074/jbc.RA118.003538
Zhang Z, Cherryholmes G, Chang F et al (2009) Evidence that cathelicidin peptide LL-37 may act as a functional ligand for CXCR2 on human neutrophils. Eur J Immunol 39:3181–3194. https://doi.org/10.1002/eji.200939496
Zhao H, Zhou J, Zhang K et al (2016) A novel peptide with potent and broad-spectrum antiviral activities against multiple respiratory viruses. Sci Rep 6:22008. https://doi.org/10.1038/srep22008
Zheng M, Gao Y, Wang G et al (2020) Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol 17:533–535. https://doi.org/10.1038/s41423-020-0402-2
Zhirnov OP, Klenk HD, Wright PF (2011) Aprotinin and similar protease inhibitors as drugs against influenza. Antiviral Res 92:27–36. https://doi.org/10.1016/j.antiviral.2011.07.014
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Rivas-Santiago, B., Jacobo-Delgado, Y. & Rodriguez-Carlos, A. Are Host Defense Peptides and Their Derivatives Ready to be Part of the Treatment of the Next Coronavirus Pandemic?. Arch. Immunol. Ther. Exp. 69, 25 (2021). https://doi.org/10.1007/s00005-021-00630-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00005-021-00630-9