Biomimetic Cascade Polymer Nanoreactors for Starvation and Photodynamic Cancer Therapy
Abstract
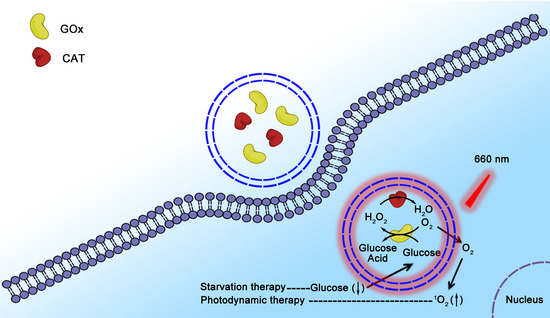
:1. Introduction
2. Results and Discussion
2.1. Characterization of Polymer Nanocapsules
2.2. Characterization of GOx/CAT-NCs
2.3. Properties of Polymer Nanocapsules and GOx/CAT-NCs
2.4. In Vitro Anticancer Effects of GOx/CAT-NCs
3. Experimental Section
3.1. Synthesis of Porphyrin-Based Building Block
3.2. Preparation of Polymer Nanocapsules and GOx/CAT-NCs
3.3. 1O2 Production Ability of Polymer Nanocapsules
3.4. Catalytic Activity Determination of GOx/CAT-NCs
3.5. Cell Culture and Cytotoxicity Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schulze, A.; Harris, A.L. How Cancer Metabolism is Tuned for Proliferation and Vulnerable to Disruption. Nature 2012, 491, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Cheong, H.; Lu, C.; Lindsten, T.; Thompson, C.B. Therapeutic Targets in Cancer Cell Metabolism and Autophagy. Nat. Biotechnol. 2012, 30, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Hay, N. Reprogramming glucose metabolism in cancer: Can it be exploited for cancer therapy? Nat. Rev. Cancer 2016, 16, 635–649. [Google Scholar] [CrossRef] [Green Version]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Oronsky, B.T.; Oronsky, N.; Fanger, G.R.; Parker, C.W.; Caroen, S.Z.; Lybeck, M.; Scicinski, J.J. Follow the ATP: Tumor energy production: A perspective. Anticancer Agents Med. Chem. 2014, 14, 1187–1198. [Google Scholar] [CrossRef]
- Tennant, D.A.; Duran, R.V.; Gottlieb, E. Targeting Metabolic Transformation for Cancer Therapy. Nat. Rev. Cancer 2010, 10, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Dang, C.V. Cancer’s Molecular Sweet Tooth and the Warburg Effect. Cancer Res. 2006, 66, 8927–8930. [Google Scholar] [CrossRef] [Green Version]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s Contributions to Current Concepts of Cancer Metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef]
- Ying, H.; Kimmelman, A.C.; Lyssiotis, C.A.; Hua, S.; Chu, G.C.; Fletcher-Sananikone, E.; Locasale, J.W.; Son, J.; Zhang, H.; Coloff, J.L.; et al. Oncogenic Kras Maintains Pancreatic Tumors through Regulation of Anabolic Glucose Metabolism. Cell 2012, 149, 656–670. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.H.; Luo, G.F.; Lei, Q.; Hong, S.; Qiu, W.X.; Liu, L.H.; Cheng, S.X.; Zhang, X.Z. Overcoming the Heat Endurance of Tumor Cells by Interfering with the Anaerobic Glycolysis Metabolism for Improved Photothermal Therapy. ACS Nano 2017, 11, 1419–1431. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kepp, O.; Vander Heiden, M.G.; Kroemer, G. Metabolic Targets for Cancer Therapy. Nat. Rev. Drug Discov. 2013, 12, 829–846. [Google Scholar] [CrossRef]
- Shibuya, K.; Okada, M.; Suzuki, S.; Seino, M.; Seino, S.; Takeda, H.; Kitanaka, C. Targeting the Facilitative Glucose Transporter GLUT1 Inhibits the Self-Renewal and Tumor-Initiating Capacity of Cancer Stem Cells. Oncotarget 2014, 6, 651–661. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Kohane, D.S. Nanoparticulate cancer-starvation therapy. Chem 2017, 2, 162–170. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Ni, D.; Liu, Y.; Yao, H.; Bu, W.; Shi, J. Magnesium silicide nanoparticles as a deoxygenation agent for cancer starvation therapy. Nat. Nanotechnol. 2017, 12, 378. [Google Scholar] [CrossRef]
- Selwan, E.M.; Finicle, B.T.; Kim, S.M.; Edinger, A.L. Attacking the Supply Wagons to Starve Cancer Cells to Death. FEBS Lett. 2016, 590, 885–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.M.; Roy, S.G.; Chen, B.; Nguyen, T.M.; McMonigle, R.J.; McCracken, A.N.; Zhang, Y.L.; Kofuji, S.; Hou, J.; Selwan, E.; et al. Targeting Cancer Metabolism by Simultaneously Disrupting Parallel Nutrient Access Pathways. J. Clin. Investig. 2016, 126, 4088–4102. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, Y.; Shi, J. Reactive oxygen species (ROS)-based nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Sibata, C.H. Oncologic photodynamic therapy photosensitizers: A clinical review. Photodiagn. Photodyn. Ther. 2010, 7, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Ochsner, M. Photophysical and Photobiological Processes in the Photodynamic Therapy of Tumours. J. Photochem. Photobiol. B 1997, 39, 1–18. [Google Scholar] [CrossRef]
- Brown, S.B.; Brown, E.A.; Walker, I. The Present and Future Role of Photodynamic Therapy in Cancer Treatment. Lancet Oncol. 2004, 5, 497–508. [Google Scholar] [CrossRef]
- Lismont, M.; Dreesen, L.; Wuttke, S. Metal-Organic Framework Nanoparticles in Photodynamic Therapy: Current Status and Perspectives. Adv. Funct. Mater. 2017, 27, 1606314. [Google Scholar] [CrossRef]
- Hota, R.; Baek, K.; Yun, G.; Kim, Y.K.; Jung, H.; Park, K.M.; Yoon, E.; Joo, T.; Kang, J.; Park, C.G.; et al. Self-Assembled, Covalently Linked, Hollow Phthalocyanine Nanospheres. Chem. Sci. 2013, 4, 339–344. [Google Scholar] [CrossRef] [Green Version]
- Roy, I.; Shetty, D.; Hota, R.; Baek, K.; Kim, J.; Kim, C.; Kappert, S.; Kim, K. A multifunctional subphthalocyanine nanosphere for targeting, labeling, and killing of antibiotic-resistant bacteria. Angew. Chem. Int. Ed. 2015, 54, 15152–15155. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xu, J.; Li, X.; Yan, T.; Yu, S.; Sun, H.; Liu, J. Template-Free Self-Assembly of Two-Dimensional Polymers into Nano/Microstructured Materials. Molecules 2021, 26, 3310. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, Z.; Li, F.; Yan, T.; Fu, S.; Tian, R.; Hou, C.; Luo, Q.; Xu, J.; Liu, J. Supramolecular polymer nanocapsules by enzymatic covalent condensation: Biocompatible and biodegradable drug-delivery systems for chemo-photothermal anticancer therapy. Polym. Chem. 2019, 10, 3566–3570. [Google Scholar] [CrossRef]
- Wang, X.R.; Hu, J.M.; Zhang, G.Y.; Liu, S.Y. Highly selective fluorogenic multianalyte biosensors constructed via enzyme-catalyzed coupling and aggregation-induced emission. J. Am. Chem. Soc. 2014, 136, 9890–9893. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Fang, Y.; Luo, Q.; Liu, S.; An, G.; Hou, C.; Lang, C.; Xu, J.; Dong, Z.; Liu, J. Construction of supramolecular polymer by enzyme-triggered covalent condensation of CB[8]-FGG-based supramonomer. Chem. Commun. 2016, 52, 2083–2086. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zou, H.; Zhang, H.; Sun, H.; Wang, T.; Pan, T.; Li, X.; Bai, Y.; Qiao, S.; Luo, Q.; et al. Enzyme-Triggered Defined Protein Nanoarrays: Efficient Light-Harvesting Systems to Mimic Chloroplasts. ACS Nano 2017, 11, 938–945. [Google Scholar] [CrossRef]
- Harms, G.S.; Pauls, S.W.; Hedstrom, J.F.; Johnson, C.K. Fluorescence and rotational dynamics of dityrosine. J. Fluoresc. 1997, 7, 283–292. [Google Scholar] [CrossRef]
- Jones, L.H.; Narayanan, A.; Hett, E.C. Understanding and applying tyrosine biochemical diversity. Mol. Biosyst. 2014, 10, 952–969. [Google Scholar] [CrossRef]
- Giulivi, C.; Traaseth, N.J.; Davies, K.J.A. Tyrosine oxidation products: Analysis and biological relevance. Amino Acids 2003, 25, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Ding, X.; Chen, L.; Wu, Y.; Liu, L.; Addicoat, M.; Irle, S.; Dong, Y.; Jiang, D. Two-dimensional artificial light-harvesting antennae with predesigned high-order structure and robust photosensitising activity. Sci. Rep. 2016, 6, 32944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiller, W.; Kliesch, H.; Wohrle, D.; Hackbarth, S.; Roder, B.; Schnurpfeil, G. Singlet Oxygen Quantum Yields of Different Photosensitizers in Polar Solvents and Micellar Solutions. J. Porphyr. Phthalocyanines 1998, 2, 145–158. [Google Scholar] [CrossRef]
- Liu, S.; Tian, R.; Xu, J.; Wang, L.; Sun, J.; Jiang, X.; Wang, T.; Li, X.; Luo, Q.; Liu, J. Cucurbit[8]uril-based supramolecular nanocapsules with a multienzyme-cascade antioxidative effect. Chem. Commun. 2019, 55, 13820–13823. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Wang, S.B.; Lei, Q.; Zhu, J.Y.; Zhang, X.Z. Ratiometric Biosensor for Aggregation-Induced Emission-Guided Precise Photodynamic Therapy. ACS Nano 2015, 9, 10268–10277. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Yan, T.; Sun, J.; Li, F.; Xu, J.; Sun, H.; Yu, S.; Liu, J. Biomimetic Cascade Polymer Nanoreactors for Starvation and Photodynamic Cancer Therapy. Molecules 2021, 26, 5609. https://doi.org/10.3390/molecules26185609
Liu S, Yan T, Sun J, Li F, Xu J, Sun H, Yu S, Liu J. Biomimetic Cascade Polymer Nanoreactors for Starvation and Photodynamic Cancer Therapy. Molecules. 2021; 26(18):5609. https://doi.org/10.3390/molecules26185609
Chicago/Turabian StyleLiu, Shengda, Tengfei Yan, Jianxin Sun, Fei Li, Jiayun Xu, Hongcheng Sun, Shuangjiang Yu, and Junqiu Liu. 2021. "Biomimetic Cascade Polymer Nanoreactors for Starvation and Photodynamic Cancer Therapy" Molecules 26, no. 18: 5609. https://doi.org/10.3390/molecules26185609