Ozone Treatment Is Insufficient to Inactivate SARS-CoV-2 Surrogate under Field Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Virus–PBS Solution
2.3. Virus–Protein Solution
2.4. Carriers
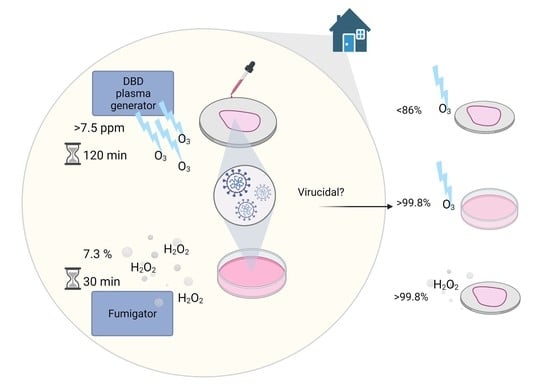
2.5. Study Design
2.6. Quantitative Carrier Tests (QCT)
2.7. Virus–PBS Solution Test
2.8. Virus Infectivity Assay
2.9. Statistical Analysis of Decontamination Efficacy
3. Results
3.1. Environmental Conditions
3.2. Ozone Treatment of Virus–PBS Solution
3.3. QCT in O3 Treatment
3.3.1. Stability of MHV on the Carriers
3.3.2. Low RH
3.3.3. High RH
3.4. QCT in H2O2 Fumigation
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meyerowitz, E.A.; Richterman, A.; Gandhi, R.T.; Sax, P.E. Transmission of SARS-CoV-2: A Review of Viral, Host, and Environmental Factors. Ann. Int. Med. 2021, 174, 69–79. [Google Scholar] [CrossRef]
- Bulfone, T.C.; Malekinejad, M.; Rutherford, G.W.; Razani, N. Outdoor Transmission of SARS-CoV-2 and Other Respiratory Viruses: A Systematic Review. J. Infect. Dis. 2021, 223, 550–561. [Google Scholar] [CrossRef]
- Noorimotlagh, Z.; Jaafarzadeh, N.; Martínez, S.S.; Mirzaee, S.A. A systematic review of possible airborne transmission of the COVID-19 virus (SARS-CoV-2) in the indoor air environment. Environ. Res. 2021, 193, 110612. [Google Scholar] [CrossRef]
- Liu, Y.; Ning, Z.; Chen, Y.; Guo, M.; Liu, Y.; Gali, N.K.; Sun, L.; Duan, Y.; Cai, J.; Westerdahl, D.; et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 2020, 582, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Razzini, K.; Castrica, M.; Menchetti, L.; Maggi, L.; Negroni, L.; Orfeo, N.V.; Pizzoccheri, A.; Stocco, M.; Muttini, S.; Balzaretti, C.M. SARS-CoV-2 RNA detection in the air and on surfaces in the COVID-19 ward of a hospital in Milan, Italy. Sci. Total Environ. 2020, 742, 140540. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Mondelli, M.U.; Colaneri, M.; Seminari, E.M.; Baldanti, F.; Bruno, R. Low risk of SARS-CoV-2 transmission by fomites in real-life conditions. Lancet Infect. Dis. 2020, 3099, 30678. [Google Scholar] [CrossRef]
- Kasloff, S.B.; Leung, A.; Strong, J.E.; Funk, D.; Cutts, T. Stability of SARS-CoV-2 on critical personal protective equipment. Sci. Rep. 2021, 11, 984. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Ren, L.; Wu, S.; Ma, W.; Yang, J.; Di, L.; Li, J.; Xiao, Y.; Kang, L.; Du, S.; et al. Cold-chain food contamination as the possible origin of COVID-19 resurgence in Beijing. Natl. Sci. Rev. 2020, 7, 1861–1864. [Google Scholar] [CrossRef]
- Brlek, A.; Vidovič, Š.; Vuzem, S.; Turk, K.; Simonović, Z. Possible indirect transmission of COVID-19 at a squash court, Slovenia, March 2020: Case report. Epidemiol. Infect. 2020, 148, e120. [Google Scholar] [CrossRef]
- Cai, J.; Sun, W.; Huang, J.; Gamber, M.; Wu, J.; He, G. Indirect Virus Transmission in Cluster of COVID-19 Cases, Wenzhou, China, 2020. Emerg. Infect. Dis. 2020, 26, 1343–1345. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D. COVID-19 rarely spreads through surfaces. So why are we still deep cleaning? Nature 2021, 590, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiling, G.; Yin, C.; Ling, W.; Xiaosong, W.; Jingjing, F.; Fang, L.; Xiaoyan, Z.; Yiyue, G.; Ying, C.; Lunbiao, C.; et al. In vitro inactivation of SARS-CoV-2 by commonly used disinfection products and methods. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Schinköthe, J.; Scheinemann, H.A.; Diederich, S.; Freese, H.; Eschbaumer, M.; Teifke, J.P.; Reiche, S. Airborne Disinfection by Dry Fogging Efficiently Inactivates Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Mycobacteria, and Bacterial Spores and Shows Limitations of Commercial Spore Carriers. Appl. Environ. Microbiol. 2020, 87, 1–14. [Google Scholar] [CrossRef]
- Grignani, E.; Mansi, A.; Cabella, R.; Castellano, P.; Tirabasso, A.; Sisto, R.; Spagnoli, M.; Fabrizi, G.; Frigerio, F.; Tranfo, G. Safe and Effective Use of Ozone as Air and Surface Disinfectant in the Conjuncture of COVID-19. Gases 2020, 1, 19–32. [Google Scholar] [CrossRef]
- Quevedo-león, R.; Bastías-Montes, J.; Espinoza-Tellez, T.; Ronceros, B.; Balic, I.; Muñoz, O. Inactivation of Coronaviruses in food industry: The use of inorganic and organic disinfectants, ozone, and UV radiation. Sci. Agropecu. 2020, 11, 257–266. [Google Scholar] [CrossRef]
- Zhou, Z.; Zuber, S.; Cantergiani, F.; Sampers, I.; Devlieghere, F.; Uyttendaele, M. Inactivation of Foodborne Pathogens and Their Surrogates on Fresh and Frozen Strawberries Using Gaseous Ozone. Front. Sustain. Food Syst. 2018, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Alimohammadi, M.; Naderi, M. Effectiveness of Ozone Gas on Airborne Virus Inactivation in Enclosed Spaces: A Review Study. Ozone Sci. Eng. 2021, 43, 21–31. [Google Scholar] [CrossRef]
- Hu, X.; Chen, Z.; Su, Z.; Deng, F.; Chen, X.; Yang, Q.; Li, P.; Chen, Q.; Ma, J.; Guan, W.; et al. Ozone Water Is an Effective Disinfectant for SARS-CoV-2. Virol. Sin. 2021, 12250, 5–7. [Google Scholar] [CrossRef]
- Zhang, D.; Ling, H.; Huang, X.; Li, J.; Li, W.; Yi, C.; Zhang, T.; Jiang, Y.; He, Y.; Deng, S.; et al. Potential spreading risks and disinfection challenges of medical wastewater by the presence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of Fangcang Hospital. Sci. Total Environ. 2020, 741, 140445. [Google Scholar] [CrossRef] [PubMed]
- Clavo, B.; Córdoba-Lanús, E.; Rodríguez-Esparragón, F.; Cazorla-Rivero, S.E.; García-Pérez, O.; Piñero, J.E.; Villar, J.; Blanco, A.; Torres-Ascensión, C.; Martín-Barrasa, J.L.; et al. Effects of Ozone Treatment on Personal Protective Equipment Contaminated with SARS-CoV-2. Antioxidants 2020, 9, 1222. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.; Atkinson, A.; Zamyadi, A.; Kibuye, F.; McKie, M.; Hogard, S.; Mollica, P.; Jasim, S.; Wert, E.C. Critical Review and Research Needs of Ozone Applications Related to Virus Inactivation: Potential Implications for SARS-CoV-2. Ozone Sci. Eng. 2021, 43, 2–20. [Google Scholar] [CrossRef]
- Criscuolo, E.; Diotti, R.A.; Ferrarese, R.; Alippi, C.; Viscardi, G.; Signorelli, C.; Mancini, N.; Clementi, M.; Clementi, N. Fast inactivation of SARS-CoV-2 by UV-C and ozone exposure on different materials. Emerg. Microbes Infect. 2021, 10, 206–210. [Google Scholar] [CrossRef]
- Moccia, G.; De Caro, F.; Pironti, C.; Boccia, G.; Capunzo, M.; Borrelli, A.; Motta, O. Development and Improvement of an Effective Method for Air and Surfaces Disinfection with Ozone Gas as a Decontaminating Agent. Medicina 2020, 56, 578. [Google Scholar] [CrossRef]
- Yano, H.; Nakano, R.; Suzuki, Y.; Nakano, A.; Kasahara, K.; Hosoi, H. Inactivation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by gaseous ozone treatment. J. Hosp. Infect. 2020, 106, 837–838. [Google Scholar] [CrossRef] [PubMed]
- Zucker, I.; Lester, Y.; Alter, J.; Werbner, M.; Yecheskel, Y.; Gal-Tanamy, M.; Dessau, M. Pseudoviruses for the assessment of coronavirus disinfection by ozone. Environ. Chem. Lett. 2021, 19, 1779–1785. [Google Scholar] [CrossRef]
- Cristiano, L. Could ozone be an effective disinfection measure against the novel coronavirus (SARS-CoV-2)? J. Prev. Med. Hyg. 2020, 61, E301–E303. [Google Scholar] [CrossRef]
- Liao, L.; Xiao, W.; Zhao, M.; Yu, X.; Wang, H.; Wang, Q.; Chu, S.; Cui, Y. Can N95 Respirators Be Reused after Disinfection? How Many Times? ACS Nano 2020, 14, 6348–6356. [Google Scholar] [CrossRef]
- British Standard Institution BS-EN 17272:2020. Chemical Disinfectants and Antiseptics—Methods of Airborne Room Disinfection by Automated Process—Determination of Bactericidal, Mycobactericidal, Sporicidal, Fungicidal, Yeasticidal, Virucidal and Phagocidal Activities; CEN-CENELEC Management Centre: Brussels, Belgium, 2020. [Google Scholar]
- Milewska, A.; Ciejka, J.; Kaminski, K.; Karewicz, A.; Bielska, D.; Zeglen, S.; Karolak, W.; Nowakowska, M.; Potempa, J.; Bosch, B.J.; et al. Novel polymeric inhibitors of HCoV-NL63. Antiviral Res. 2013, 97, 112–121. [Google Scholar] [CrossRef] [Green Version]
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Dempsey, D.M.; Dutilh, B.E.; Harrach, B.; Harrison, R.L.; Hendrickson, R.C.; et al. Changes to virus taxonomy and the Statutes ratified by the International Committee on Taxonomy of Viruses (2020). Arch. Virol. 2020, 165, 2737–2748. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Bertsch, P.M.; Bivins, A.; Bibby, K.; Farkas, K.; Gathercole, A.; Haramoto, E.; Gyawali, P.; Korajkic, A.; McMinn, B.R.; et al. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020, 739, 139960. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Bertsch, P.M.; Bibby, K.; Haramoto, E.; Hewitt, J.; Huygens, F.; Gyawali, P.; Korajkic, A.; Riddell, S.; Sherchan, S.P.; et al. Decay of SARS-CoV-2 and surrogate murine hepatitis virus RNA in untreated wastewater to inform application in wastewater-based epidemiology. Environ. Res. 2020, 191, 110092. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.L.; Xiang, J.; Mackin, S.R.; Perlman, S.; Thorne, P.; O’Shaughnessy, P.; Strzelecki, B.; Aubin, P.; Ortiz-Hernandez, M.; Stapleton, J.T. Inactivation of Severe Acute Respiratory Coronavirus Virus 2 (SARS-CoV-2) and Diverse RNA and DNA Viruses on Three-Dimensionally Printed Surgical Mask Materials. Infect. Control Hosp. Epidemiol. 2021, 42, 253–260. [Google Scholar] [CrossRef]
- Lee, J.; Bong, C.; Lim, W.; Bae, P.K.; Abafogi, A.T.; Baek, S.H.; Shin, Y.B.; Bak, M.S.; Park, S. Fast and Easy Disinfection of Coronavirus-Contaminated Face Masks Using Ozone Gas Produced by a Dielectric Barrier Discharge Plasma Generator. Environ. Sci. Technol. Lett. 2021, 8, 339–344. [Google Scholar] [CrossRef]
- Murata, T.; Komoto, S.; Iwahori, S.; Sasaki, J.; Nishitsuji, H.; Hasebe, T.; Hoshinaga, K.; Yuzawa, Y. Reduction of severe acute respiratory syndrome coronavirus-2 infectivity by admissible concentration of ozone gas and water. Microbiol. Immunol. 2021, 65, 10–16. [Google Scholar] [CrossRef]
- Percivalle, E.; Clerici, M.; Cassaniti, I.; Vecchio Nepita, E.; Marchese, P.; Olivati, D.; Catelli, C.; Berri, A.; Baldanti, F.; Marone, P.; et al. SARS-CoV-2 viability on different surfaces after gaseous ozone treatment: A preliminary evaluation. J. Hosp. Infect. 2021, 110, 33–36. [Google Scholar] [CrossRef]
- Uppal, T.; Khazaieli, A.; Snijders, A.M.; Verma, S.C. Inactivation of Human Coronavirus by FATHHOME’s Dry Sanitizer Device: Rapid and Eco-Friendly Ozone-Based Disinfection of SARS-CoV-2. Pathogens 2021, 10, 339. [Google Scholar] [CrossRef]
- Juszkiewicz, M.; Walczak, M.; Mazur-Panasiuk, N.; Woźniakowski, G. Effectiveness of Chemical Compounds Used against African Swine Fever Virus in Commercial Available Disinfectants. Pathogens 2020, 9, 878. [Google Scholar] [CrossRef]
- Biryukov, J.; Boydston, J.A.; Dunning, R.A.; Yeager, J.J.; Wood, S.; Reese, A.L.; Ferris, A.; Miller, D.; Weaver, W.; Zeitouni, N.E.; et al. Increasing Temperature and Relative Humidity Accelerates Inactivation of SARS-CoV-2 on Surfaces. MSphere 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Casanova, L.M.; Jeon, S.; Rutala, W.A.; Weber, D.J.; Sobsey, M.D. Effects of Air Temperature and Relative Humidity on Coronavirus Survival on Surfaces. Appl. Environ. Microbiol. 2010, 76, 2712–2717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubuis, M.-E.; Dumont-Leblond, N.; Laliberté, C.; Veillette, M.; Turgeon, N.; Jean, J.; Duchaine, C. Ozone efficacy for the control of airborne viruses: Bacteriophage and norovirus models. PLoS ONE 2020, 15, e0231164. [Google Scholar] [CrossRef] [Green Version]
- Yin, S.-E.; Sun, B.-M.; Gao, X.-D.; Xiao, H.-P. The Effect of Oxygen and Water Vapor on Nitric Oxide Conversion with a Dielectric Barrier Discharge Reactor. Plasma Chem. Plasma Process. 2009, 29, 421–431. [Google Scholar] [CrossRef]
- Unger-Bimczok, B.; Kottke, V.; Hertel, C.; Rauschnabel, J. The Influence of Humidity, Hydrogen Peroxide Concentration, and Condensation on the Inactivation of Geobacillus stearothermophilus Spores with Hydrogen Peroxide Vapor. J. Pharm. Innov. 2008, 3, 123–133. [Google Scholar] [CrossRef]
- Dee, S.A.; Bauermann, F.V.; Niederwerder, M.C.; Singrey, A.; Clement, T.; de Lima, M.; Long, C.; Patterson, G.; Sheahan, M.A.; Stoian, A.M.M.; et al. Survival of viral pathogens in animal feed ingredients under transboundary shipping models. PLoS ONE 2018, 13, e0194509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, H.; Song, Y.; Chen, Y.; Wu, N.; Xu, J.; Sun, C.; Zhang, J.; Weng, T.; Zhang, Z.; Wu, Z.; et al. Molecular Architecture of the SARS-CoV-2 Virus. Cell 2020, 183, 730–738.e13. [Google Scholar] [CrossRef] [PubMed]
- Barcena, M.; Oostergetel, G.T.; Bartelink, W.; Faas, F.G.A.; Verkleij, A.; Rottier, P.J.M.; Koster, A.J.; Bosch, B.J. Cryo-electron tomography of mouse hepatitis virus: Insights into the structure of the coronavirion. Proc. Natl. Acad. Sci. USA 2009, 106, 582–587. [Google Scholar] [CrossRef] [Green Version]





| Unit Number | Unit Name * | Pretreatment | Conditions after Pretreatment | Treatment | Conditions after Treatment |
|---|---|---|---|---|---|
| 1 | Untreated + Low RH | No | RH = 21.8% | No | RH = 21.8% |
| 2 | Treated O3 + Low RH | 60 min O3 | RH = 21.8% O3 > 7.3 ppm | 120 min O3 | RH = 21.8% O3 > 7.3 ppm |
| 3 | Untreated + High RH | 60 min humidification | RH = 49.8% | 120 min humidification | RH = 54.2% |
| 4 | Treated O3 + High RH | 60 min humidification 60 min O3 | RH = 49.8% O3 > 7.3 ppm | 120 min O3120 min humidification | RH = 54.2% O3 > 7.3 ppm |
| 5 | Treated H2O2 + Low RH | No | RH = 21.8% | 30 min H2O2 | RH = n/a H2O2 = n/a |
| QCTs | LRV between Untreated and Treated Samples | LRV between the Initial Titre and Treated Samples | ||||||||
| Ozone | Hydrogen Peroxide | Ozone | Hydrogen Peroxide | |||||||
| Timepoint (min) | Low RH | High RH | Timepoint (min) | Low RH | Timepoint (min) | Low RH | High RH | Timepoint (min) | Low RH | |
| 0 | 0.0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0.0 | 0 | 0.0 | |
| 60 | 0.0 | 0.0 | 60 | 0.0 | 60 | 0.9 | 0.6 | 60 | 0.2 | |
| 75 | +0.2 | 0.6 | 65 | 1.5 (***) | 75 | 0.6 | 1.5 (***) | 65 | 1.8 (***) | |
| 90 | 0.2 | +0.3 | 70 | 2.2 (***) | 90 | 0.7 | 1.0 (*) | 70 | 2.5 (***) | |
| 105 | +0.3 | 0.3 | 80 | 2.6 (***) | 105 | 0.0 | 1.0 (*) | 80 | 2.9 (***) | |
| 120 | +0.5 | 0.8 | 90 | 2.9 (***) | 120 | 0.1 | 1.4 (***) | 90 | 3.3 (***) | |
| 150 | 0.3 | 0.4 | 150 | 0.6 | 1.5 (***) | |||||
| 180 | 0.3 | 0.8 (*) | 180 | 0.7 | 2.1 (***) | |||||
| Virus Suspension | 0 | 0.0 | n/a | n/a | n/a | 0 | 0.0 | n/a | n/a | n/a |
| 5 | 1.2 | n/a | n/a | n/a | 5 | 2.1 (***) | n/a | n/a | n/a | |
| 15 | 1.7 (**) | n/a | n/a | n/a | 15 | 2.5 (***) | n/a | n/a | n/a | |
| 150 | 3.0 (***) | n/a | n/a | n/a | 150 | 3.3 (***) | n/a | n/a | n/a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazur-Panasiuk, N.; Botwina, P.; Kutaj, A.; Woszczyna, D.; Pyrc, K. Ozone Treatment Is Insufficient to Inactivate SARS-CoV-2 Surrogate under Field Conditions. Antioxidants 2021, 10, 1480. https://doi.org/10.3390/antiox10091480
Mazur-Panasiuk N, Botwina P, Kutaj A, Woszczyna D, Pyrc K. Ozone Treatment Is Insufficient to Inactivate SARS-CoV-2 Surrogate under Field Conditions. Antioxidants. 2021; 10(9):1480. https://doi.org/10.3390/antiox10091480
Chicago/Turabian StyleMazur-Panasiuk, Natalia, Pawel Botwina, Adrian Kutaj, Damian Woszczyna, and Krzysztof Pyrc. 2021. "Ozone Treatment Is Insufficient to Inactivate SARS-CoV-2 Surrogate under Field Conditions" Antioxidants 10, no. 9: 1480. https://doi.org/10.3390/antiox10091480