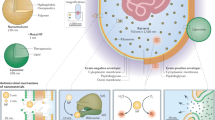
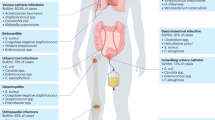
Abstract
The rise in antibiotic-resistant bacteria, including strains that are resistant to last-resort antibiotics, and the limited ability of antibiotics to eradicate biofilms have necessitated the development of alternative antibacterial therapeutics. Antibacterial biomaterials, such as polycationic polymers, and biomaterial-assisted delivery of non-antibiotic therapeutics, such as bacteriophages, antimicrobial peptides and antimicrobial enzymes, have improved our ability to treat antibiotic-resistant and recurring infections. Biomaterials not only allow targeted delivery of multiple agents but also sustained release at the infection site, thereby reducing potential systemic adverse effects. In this Review, we discuss biomaterial-based non-antibiotic antibacterial therapies for the treatment of community-acquired and hospital-acquired infectious diseases, with a focus on in vivo results. We highlight the translational potential of different biomaterial-based strategies and provide a perspective on the challenges associated with their clinical translation. Finally, we discuss the future scope of biomaterial-assisted antibacterial therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Liu, J., Gefen, O., Ronin, I., Bar-Meir, M. & Balaban, N. Q. Effect of tolerance on the evolution of antibiotic resistance under drug combinations. Science 367, 200–204 (2020).
Costerton, J. W., Stewart, P. S. & Greenberg, E. P. Bacterial biofilms: a common cause of persistent infections. Science 284, 1318–1322 (1999).
Koo, H., Allan, R. N., Howlin, R. P., Stoodley, P. & Hall-Stoodley, L. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat. Rev. Microbiol. 15, 740–755 (2017).
Pumart, P. et al. Health and economic impacts of antimicrobial resistance in Thailand. J. Health Serv. Res. Policy 6, 352–360 (2012).
Sprenger, M. & Fukuda, K. New mechanisms, new worries. Science 351, 1263–1264 (2016).
Edelstein, M. V. et al. Spread of extensively resistant VIM-2-positive ST235 Pseudomonas aeruginosa in Belarus, Kazakhstan, and Russia: a longitudinal epidemiological and clinical study. Lancet Infect. Dis. 13, 867–876 (2013).
Weiner-Lastinger, L. M. et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect. Control Hosp. Epidemiol. 41, 1–18 (2020).
Weiner-Lastinger, L. M. et al. Antimicrobial-resistant pathogens associated with pediatric healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect. Control Hosp. Epidemiol. 41, 19–30 (2020).
Weiner, L. M. et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control Hosp. Epidemiol. 37, 1288–1301 (2016).
Levin-Reisman, I. et al. Antibiotic tolerance facilitates the evolution of resistance. Science 355, 826–830 (2017).
Langdon, A., Crook, N. & Dantas, G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 8, 39 (2016).
van Santen, K. L. et al. The standardized antimicrobial administration ratio: a new metric for measuring and comparing antibiotic use. Clin. Infect. Dis. 67, 179–185 (2018).
Dedrick, R. M. et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 25, 730–733 (2019).
Schuch, R., Nelson, D. & Fischetti, V. A. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature 418, 884–889 (2002).
Loeffler, J. M. & Fischetti, V. A. Synergistic lethal effect of a combination of phage lytic enzymes with different activities on penicillin-sensitive and -resistant Streptococcus pneumoniae strains. Antimicrob. Agents Chemother. 47, 375–377 (2003).
Chen, C. H. & Lu, T. K. Development and challenges of antimicrobial peptides for therapeutic applications. Antibiotics 9, 24 (2020).
Usmani, S. S. et al. THPdb: Database of FDA-approved peptide and protein therapeutics. PLoS ONE 12, e0181748 (2017).
Donlan, R. M. Preventing biofilms of clinically relevant organisms using bacteriophage. Trends Microbiol. 17, 66–72 (2009).
Labrie, S. J., Samson, J. E. & Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 8, 317–327 (2010).
Dufour, N., Delattre, R., Ricard, J. D. & Debarbieux, L. The lysis of pathogenic Escherichia coli by bacteriophages releases less endotoxin than by β-lactams. Clin. Infect. Dis. 64, 1582–1588 (2017).
Cho, I. & Blaser, M. J. The human microbiome: at the interface of health and disease. Nat. Rev. Genet. 13, 260–270 (2012).
Schooley, R. T. et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob. Agents Chemother. 61, e00954-17 (2017).
Jennes, S. et al. Use of bacteriophages in the treatment of colistin-only-sensitive Pseudomonas aeruginosa septicaemia in a patient with acute kidney injury — a case report. Crit. Care 21, 129 (2017).
Wroe, J. A., Johnson, C. T. & García, A. J. Bacteriophage delivering hydrogels reduce biofilm formation in vitro and infection in vivo. J. Biomed. Mater. Res. A 108, 39–49 (2020).
Meurice, E. et al. New antibacterial microporous CaP materials loaded with phages for prophylactic treatment in bone surgery. J. Mater. Sci. Mater. Med. 23, 2445–2452 (2012).
Barros, J. A. R. et al. Encapsulated bacteriophages in alginate-nanohydroxyapatite hydrogel as a novel delivery system to prevent orthopedic implant-associated infections. Nanomedicine 24, 102145 (2020).
Kaur, S., Harjai, K. & Chhibber, S. In vivo assessment of phage and linezolid based implant coatings for treatment of methicillin resistant S. aureus (MRSA) mediated orthopaedic device related infections. PLoS ONE 11, e0157626 (2016).
Carrigy, N. B. et al. Prophylaxis of Mycobacterium tuberculosis H37Rv infection in a preclinical mouse model via inhalation of nebulized bacteriophage D29. Antimicrob. Agents Chemother. 63, e00871-19 (2019).
Prazak, J. et al. Nebulized bacteriophages for prophylaxis of experimental ventilator-associated pneumonia due to methicillin-resistant Staphylococcus aureus. Crit. Care Med. 48, 1042–1046 (2020).
Golshahi, L., Lynch, K. H., Dennis, J. J. & Finlay, W. H. In vitro lung delivery of bacteriophages KS4-M and ΦKZ using dry powder inhalers for treatment of Burkholderia cepacia complex and Pseudomonas aeruginosa infections in cystic fibrosis. J. Appl. Microbiol. 110, 106–117 (2011).
Singla, S., Harjai, K., Katare, O. P. & Chhibber, S. Bacteriophage-loaded nanostructured lipid carrier: improved pharmacokinetics mediates effective resolution of Klebsiella pneumoniae-induced lobar pneumonia. J. Infect. Dis. 212, 325–334 (2015).
Agarwal, R. et al. Inhaled bacteriophage-loaded polymeric microparticles ameliorate acute lung infections. Nat. Biomed. Eng. 2, 841–849 (2018). Polymeric microparticles facilitate delivery of bacteriophages to mitigate bacterial lung infections in wild-type and cystic fibrosis transgenic mice.
Vinner, G. K., Richards, K., Leppanen, M., Sagona, A. P. & Malik, D. J. Microencapsulation of enteric bacteriophages in a pH-responsive solid oral dosage formulation using a scalable membrane emulsification process. Pharmaceutics 11, 475 (2019).
Vinner, G. K., Vladisavljević, G. T., Clokie, M. R. J. & Malik, D. J. Microencapsulation of Clostridium difficile specific bacteriophages using microfluidic glass capillary devices for colon delivery using pH triggered release. PLoS ONE 12, e0186239 (2017).
Thakral, S., Thakral, N. K. & Majumdar, D. K. Eudragit®: a technology evaluation. Expert Opin. Drug Deliv. 10, 131–149 (2013).
Ma, Y. P. et al. Enhanced alginate microspheres as means of oral delivery of bacteriophage for reducing Staphylococcus aureus intestinal carriage. Food Hydrocoll. 26, 434–440 (2012).
Colom, J. et al. Microencapsulation with alginate/CaCO3: A strategy for improved phage therapy. Sci. Rep. 7, 41441 (2017).
Adamu Ahmad, K., Sabo Mohammed, A. & Abas, F. Chitosan nanoparticles as carriers for the delivery of ΦKAZ14 bacteriophage for oral biological control of colibacillosis in chickens. Molecules 21, 256 (2016).
Rastogi, V., Yadav, P., Verma, A. & Pandit, J. K. Ex vivo and in vivo evaluation of microemulsion based transdermal delivery of E. coli specific T4 bacteriophage: A rationale approach to treat bacterial infection. Eur. J. Pharm. Sci. 107, 168–182 (2017).
Jain, S., Chaudhari, B. H. & Swarnakar, N. K. Preparation and characterization of niosomal gel for iontophoresis mediated transdermal delivery of isosorbide dinitrate. Drug Deliv. Transl Res. 1, 309–321 (2011).
Sarhan, W. A. & Azzazy, H. M. Apitherapeutics and phage-loaded nanofibers as wound dressings with enhanced wound healing and antibacterial activity. Nanomedicine 12, 2055–2067 (2017).
Cheng, W. et al. Incorporation of bacteriophages in polycaprolactone/collagen fibers for antibacterial hemostatic dual-function. J. Biomed. Mater. Res. Part B 106, 2588–2595 (2018).
Chhibber, S., Kaur, J. & Kaur, S. Liposome entrapment of bacteriophages improves wound healing in a diabetic mouse MRSA infection. Front. Microbiol. 9, 561 (2018).
Chadha, P., Katare, O. P. & Chhibber, S. Liposome loaded phage cocktail: Enhanced therapeutic potential in resolving Klebsiella pneumoniae mediated burn wound infections. Burns 43, 1532–1543 (2017).
Rubalskii, E. et al. Fibrin glue as a local drug-delivery system for bacteriophage PA5. Sci. Rep. 9, 2091 (2019).
Centers for Disease Control and Prevention. Catheter-associated urinary tract infections (CAUTI). CDC https://www.cdc.gov/hai/ca_uti/uti.html (2015).
Lehman, S. M. & Donlan, R. M. Bacteriophage-mediated control of a two-species biofilm formed by microorganisms causing catheter-associated urinary tract infections in an in vitro urinary catheter model. Antimicrob. Agents Chemother. 59, 1127–1137 (2015).
Liao, K. S., Lehman, S. M., Tweardy, D. J., Donlan, R. M. & Trautner, B. W. Bacteriophages are synergistic with bacterial interference for the prevention of Pseudomonas aeruginosa biofilm formation on urinary catheters. J. Appl. Microbiol. 113, 1530–1539 (2012).
Milo, S. et al. Prevention of encrustation and blockage of urinary catheters by Proteus mirabilis via pH-triggered release of bacteriophage. J. Mater. Chem. B 5, 5403–5411 (2017).
Lungren, M. P. et al. Bacteriophage K antimicrobial-lock technique for treatment of Staphylococcus aureus central venous catheter-related infection: a leporine model efficacy analysis. J. Vasc. Interv. Radiol. 25, 1627–1632 (2014).
Curtin, J. J. & Donlan, R. M. Using bacteriophages to reduce formation of catheter-associated biofilms by Staphylococcus epidermidis. Antimicrob. Agents Chemother. 50, 1268–1275 (2006).
Fu, W. et al. Bacteriophage cocktail for the prevention of biofilm formation by Pseudomonas aeruginosa on catheters in an in vitro model system. Antimicrob. Agents Chemother. 54, 397–404 (2010).
Mookherjee, N., Anderson, M. A., Haagsman, H. P. & Davidson, D. J. Antimicrobial host defence peptides: functions and clinical potential. Nat. Rev. Drug Discov. 19, 311–332 (2020).
Di, Y. P. et al. Enhanced therapeutic index of an antimicrobial peptide in mice by increasing safety and activity against multidrug-resistant bacteria. Sci. Adv. 6, eaay6817 (2020).
Lazzaro, B. P., Zasloff, M. & Rolff, J. Antimicrobial peptides: Application informed by evolution. Science 368, eaau5480 (2020).
Gordon, Y. J., Romanowski, E. G. & McDermott, A. M. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr. Eye Res. 30, 505–515 (2005).
Bacalum, M. & Radu, M. Cationic antimicrobial peptides cytotoxicity on mammalian cells: an analysis using therapeutic index integrative concept. Int. J. Pept. Res. Ther. 21, 47–55 (2015).
Rai, A. et al. One-step synthesis of high-density peptide-conjugated gold nanoparticles with antimicrobial efficacy in a systemic infection model. Biomaterials 85, 99–110 (2016).
Qi, G. B., Zhang, D., Liu, F. H., Qiao, Z. Y. & Wang, H. An “on-site transformation” strategy for treatment of bacterial infection. Adv. Mater. 29, 1703461 (2017).
Kwon, E. J. et al. Porous silicon nanoparticle delivery of tandem peptide anti-infectives for the treatment of Pseudomonas aeruginosa lung infections. Adv. Mater. 29, 1701527 (2017).
Lim, K. et al. Anhydrous polymer-based coating with sustainable controlled release functionality for facile, efficacious impregnation, and delivery of antimicrobial peptides. Biotechnol. Bioeng. 115, 2000–2012 (2018).
Qi, F. et al. Practical preparation of infection-resistant biomedical surfaces from antimicrobial β-peptide polymers. ACS Appl. Mater. Interfaces 11, 18907–18913 (2019).
Zhuk, I. et al. Self-defensive layer-by-layer films with bacteria-triggered antibiotic release. ACS Nano 8, 7733–7745 (2014).
Zhang, X.-Y. et al. Antimicrobial peptide-conjugated hierarchical antifouling polymer brushes for functionalized catheter surfaces. Biomacromolecules 20, 4171–4179 (2019).
Yu, K. et al. Anti-adhesive antimicrobial peptide coating prevents catheter associated infection in a mouse urinary infection model. Biomaterials 116, 69–81 (2017).
Gao, Q. et al. Rationally designed dual functional block copolymers for bottlebrush-like coatings: In vitro and in vivo antimicrobial, antibiofilm, and antifouling properties. Acta Biomater. 51, 112–124 (2017).
Chen, R., Willcox, M. D., Ho, K. K., Smyth, D. & Kumar, N. Antimicrobial peptide melimine coating for titanium and its in vivo antibacterial activity in rodent subcutaneous infection models. Biomaterials 85, 142–151 (2016).
Shen, X. et al. Antibacterial and osteogenesis performances of LL37-loaded titania nanopores in vitro and in vivo. Int. J. Nanomed. 14, 3043–3054 (2019).
Song, Y.-Y., Schmidt-Stein, F., Bauer, S. & Schmuki, P. Amphiphilic TiO2 nanotube arrays: an actively controllable drug delivery system. J. Am. Chem. Soc. 131, 4230–4232 (2009).
Kazemzadeh-Narbat, M. et al. Multilayered coating on titanium for controlled release of antimicrobial peptides for the prevention of implant-associated infections. Biomaterials 34, 5969–5977 (2013).
Shi, J. et al. Biological and immunotoxicity evaluation of antimicrobial peptide-loaded coatings using a layer-by-layer process on titanium. Sci. Rep. 5, 16336 (2015).
Kazemzadeh-Narbat, M. et al. Drug release and bone growth studies of antimicrobial peptide-loaded calcium phosphate coating on titanium. J. Biomed. Mater. Res. Part B 100, 1344–1352 (2012).
Yang, G. et al. Sustained release of antimicrobial peptide from self-assembling hydrogel enhanced osteogenesis. J. Biomater. Sci. Polym. Ed. 29, 1812–1824 (2018).
Yuan, X. et al. Multifunctional sulfonated polyetheretherketone coating with beta-defensin-14 for yielding durable and broad-spectrum antibacterial activity and osseointegration. Acta Biomater. 86, 323–337 (2019).
Cormier, A. R., Pang, X., Zimmerman, M. I., Zhou, H.-X. & Paravastu, A. K. Molecular structure of RADA16-I designer self-assembling peptide nanofibers. ACS Nano 7, 7562–7572 (2013).
Briuglia, M. L., Urquhart, A. J. & Lamprou, D. A. Sustained and controlled release of lipophilic drugs from a self-assembling amphiphilic peptide hydrogel. Int. J. Pharm. 474, 103–111 (2014).
Irwansyah, I. et al. Gram-positive antimicrobial activity of amino acid-based hydrogels. Adv. Mater. 27, 648–654 (2015).
Lohmann, N. et al. Glycosaminoglycan-based hydrogels capture inflammatory chemokines and rescue defective wound healing in mice. Sci. Transl Med. 9, eaai9044 (2017).
Li, J., Liang, S., Yan, Y., Tian, X. & Li, X. O-mannosylation affords a glycopeptide hydrogel with inherent antibacterial activities against E. coli via multivalent interactions between lectins and supramolecular assemblies. Macromol. Biosci. 19, e1900124 (2019).
Xie, Z. et al. Design of antimicrobial peptides conjugated biodegradable citric acid derived hydrogels for wound healing. J. Biomed. Mater. Res. A 103, 3907–3918 (2015).
Liu, M. et al. Fabrication of KR-12 peptide-containing hyaluronic acid immobilized fibrous eggshell membrane effectively kills multi-drug-resistant bacteria, promotes angiogenesis and accelerates re-epithelialization. Int. J. Nanomed. 14, 3345–3360 (2019).
Obuobi, S. et al. Facile and efficient encapsulation of antimicrobial peptides via crosslinked DNA nanostructures and their application in wound therapy. J. Control. Rel. 313, 120–130 (2019).
Ch’ng, J.-H., Chong, K. K. L., Lam, L. N., Wong, J. J. & Kline, K. A. Biofilm-associated infection by enterococci. Nat. Rev. Microbiol. 17, 82–94 (2019).
Wolcott, R. D., Rhoads, D. D. & Dowd, S. E. Biofilms and chronic wound inflammation. J. Wound Care 17, 333–341 (2008).
Maiden, M. M., Zachos, M. P. & Waters, C. M. Hydrogels embedded with melittin and tobramycin are effective against Pseudomonas aeruginosa biofilms in an animal wound model. Front. Microbiol. 10, 1348 (2019).
Wang, J. et al. pH-Switchable antimicrobial nanofiber networks of hydrogel eradicate biofilm and rescue stalled healing in chronic wounds. ACS Nano 13, 11686–11697 (2019). A pH-responsive hydrogel that displays antimicrobial activity at an acidic pH, which is characteristic for the pathological environment of infected chronic wounds, erradicates biofilms and facilitates wound healing.
Puthia, M. et al. A dual-action peptide-containing hydrogel targets wound infection and inflammation. Sci. Transl Med. 12, eaax6601 (2020). A TCP-25-loaded hydrogel reduces bacterial counts and inflammation in murine subcutaneous wound and porcine partial thickness wound models, and improves wound healing.
Dutta, D., Ozkan, J. & Willcox, M. D. P. Biocompatibility of antimicrobial melimine lenses: rabbit and human studies. Optom. Vis. Sci. 91, 570–581 (2014).
Cole, N. et al. In vivo performance of melimine as an antimicrobial coating for contact lenses in models of CLARE and CLPU. Invest. Ophthalmol. Vis. Sci. 51, 390–395 (2010).
Dutta, D., Vijay, A. K., Kumar, N. & Willcox, M. D. Melimine-coated antimicrobial contact lenses reduce microbial keratitis in an animal model. Invest. Ophthalmol. Vis. Sci. 57, 5616–5624 (2016).
Dutta, D. et al. Development of silicone hydrogel antimicrobial contact lenses with Mel4 peptide coating. Optom. Vis. Sci. 95, 937–946 (2018).
Gonzalez-Delgado, L. S. et al. Two-site recognition of Staphylococcus aureus peptidoglycan by lysostaphin SH3b. Nat. Chem. Biol. 16, 24–30 (2020).
Cui, F. et al. Development of chitosan-collagen hydrogel incorporated with lysostaphin (CCHL) burn dressing with anti-methicillin-resistant Staphylococcus aureus and promotion wound healing properties. Drug Deliv. 18, 173–180 (2011).
Windolf, C. D., Lögters, T., Scholz, M., Windolf, J. & Flohé, S. Lysostaphin-coated titan-implants preventing localized osteitis by Staphylococcus aureus in a mouse model. PLoS ONE 9, e115940 (2014).
Xue, B. et al. A novel controlled-release system for antibacterial enzyme lysostaphin delivery using hydroxyapatite/chitosan composite bone cement. PLoS ONE 9, e113797 (2014).
Nithya, S. et al. Preparation, characterization and efficacy of lysostaphin-chitosan gel against Staphylococcus aureus. Int. J. Biol. Macromol. 110, 157–166 (2018).
Abulateefeh, S. R. et al. Facile synthesis of responsive nanoparticles with reversible, tunable and rapid thermal transitions from biocompatible constituents. Chem. Commun. https://doi.org/10.1039/B911986H (2009).
Guo, S. et al. Engineered living materials based on adhesin-mediated trapping of programmable cells. ACS Synth. Biol. 9, 475–485 (2020).
Johnson, C. T. et al. Hydrogel delivery of lysostaphin eliminates orthopedic implant infection by Staphylococcus aureus and supports fracture healing. Proc. Natl Acad. Sci. USA 115, E4960–E4969 (2018). Hydrogel-based lysostaphin delivery completely clears orthopaedic implant infection caused by S. aureus, outperforming standard-of-care antibiotic therapy, and restores complete fracture healing in mice.
Johnson, C. T. et al. Lysostaphin and BMP-2 co-delivery reduces S. aureus infection and regenerates critical-sized segmental bone defects. Sci. Adv. 5, eaaw1228 (2019). Hydrogel-enabled co-delivery of lysostaphin and bone morphogenetic protein 2 eliminates S. aureus infection, promotes bone regeneration to bridge a segmental bone defect and restores the environment at the site of infection to a healthy (non-infected) microenvironment in mice.
Nelson, D., Loomis, L. & Fischetti, V. A. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc. Natl Acad. Sci. USA 98, 4107–4112 (2001).
Portilla, S., Fernández, L., Gutiérrez, D., Rodríguez, A. & García, P. Encapsulation of the antistaphylococcal endolysin LysRODI in pH-sensitive liposomes. Antibiotics 9, 242 (2020).
Gondil, V. S. et al. Comprehensive evaluation of chitosan nanoparticle based phage lysin delivery system; a novel approach to counter S. pneumoniae infections. Int. J. Pharm. 573, 118850 (2020).
Liu, S.-y et al. Antimicrobial activity of a quaternary ammonium methacryloxy silicate-containing acrylic resin: a randomised clinical trial. Sci. Rep. 6, 21882 (2016).
Atar-Froyman, L. et al. Anti-biofilm properties of wound dressing incorporating nonrelease polycationic antimicrobials. Biomaterials 46, 141–148 (2015).
Hoque, J., Akkapeddi, P., Ghosh, C., Uppu, D. S. S. M. & Haldar, J. A biodegradable polycationic paint that kills bacteria in vitro and in vivo. ACS Appl. Mater. Interfaces 8, 29298–29309 (2016).
Liu, L. et al. Self-assembled cationic peptide nanoparticles as an efficient antimicrobial agent. Nat. Nanotechnol. 4, 457–463 (2009).
Chen, Y. et al. Design and synthesis of biocompatible, hemocompatible, and highly selective antimicrobial cationic peptidopolysaccharides via click chemistry. Biomacromolecules 20, 2230–2240 (2019).
Andrén, O. C. J. et al. Antibiotic-free cationic dendritic hydrogels as surgical-site-infection-inhibiting coatings. Adv. Healthc. Mater. 8, e1801619 (2019).
Venkatesh, M. et al. Antimicrobial activity and cell selectivity of synthetic and biosynthetic cationic polymers. Antimicrob. Agents Chemother. 61, e00469-17 (2017).
Nederberg, F. et al. Biodegradable nanostructures with selective lysis of microbial membranes. Nat. Chem. 3, 409–414 (2011).
Li, J. et al. Block copolymer nanoparticles remove biofilms of drug-resistant gram-positive bacteria by nanoscale bacterial debridement. Nano Lett. 18, 4180–4187 (2018). Nanoparticles facilitate biofilm removal through a process of nanoscale debridement, which is orthogonal to conventional development of resistance trait in bacteria and would have widespread application in treating resistant as well as sensitive strains of bacteria.
Rahman, M. A. et al. Macromolecular-clustered facial amphiphilic antimicrobials. Nat. Commun. 9, 5231 (2018).
Lienkamp, K. et al. Antimicrobial polymers prepared by ROMP with unprecedented selectivity: a molecular construction kit approach. J. Am. Chem. Soc. 130, 9836–9843 (2008).
Ilker, M. F., Nüsslein, K., Tew, G. N. & Coughlin, E. B. Tuning the hemolytic and antibacterial activities of amphiphilic polynorbornene derivatives. J. Am. Chem. Soc. 126, 15870–15875 (2004).
Engler, A. C. et al. Antimicrobial polycarbonates: investigating the impact of balancing charge and hydrophobicity using a same-centered polymer approach. Biomacromolecules 14, 4331–4339 (2013).
Chin, W. et al. A macromolecular approach to eradicate multidrug resistant bacterial infections while mitigating drug resistance onset. Nat. Commun. 9, 917 (2018).
Lam, S. J. et al. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat. Microbiol. 1, 16162 (2016). Structurally nanoengineered AMP polymers display potent activity against a variety of Gram-negative bacteria, including colistin-resistant and multidrug-resistant pathogens, with low cytotoxicity and minimal development of resistance.
Wang, Y., Yang, Y., Shi, Y., Song, H. & Yu, C. Antibiotic-free antibacterial strategies enabled by nanomaterials: progress and perspectives. Adv. Mater. 32, 1904106 (2020).
Kirk, J. A. et al. New class of precision antimicrobials redefines role of Clostridium difficile S-layer in virulence and viability. Sci. Transl Med. 9, eaah6813 (2017).
Arifuzzaman, M. et al. MRGPR-mediated activation of local mast cells clears cutaneous bacterial infection and protects against reinfection. Sci. Adv. 5, eaav0216 (2019).
Ram, G., Ross, H. F., Novick, R. P., Rodriguez-Pagan, I. & Jiang, D. Conversion of staphylococcal pathogenicity islands to CRISPR-carrying antibacterial agents that cure infections in mice. Nat. Biotechnol. 36, 971–976 (2018).
Hwang, G. et al. Catalytic antimicrobial robots for biofilm eradication. Sci. Robot. 4, eaaw2388 (2019). Magnetically driven, catalytic antimicrobial robots efficiently and controllably kill, degrade and remove biofilms, and can be developed to fight persistent biofilm infections or mitigate biofouling of medical devices and diverse surfaces.
Qiao, Y. et al. Treatment of MRSA-infected osteomyelitis using bacterial capturing, magnetically targeted composites with microwave-assisted bacterial killing. Nat. Commun. 11, 4446 (2020).
Si, Y. et al. Daylight-driven rechargeable antibacterial and antiviral nanofibrous membranes for bioprotective applications. Sci. Adv. 4, eaar5931 (2018).
Berry, G. C., Bockstaller, M. R. & Matyjaszewski, K. Celebrating 100 years of polymer science. Prog. Polym. Sci. 100, 101193 (2020).
Zhang, L. et al. Self-assembled lipid–polymer hybrid nanoparticles: a robust drug delivery platform. ACS Nano 2, 1696–1702 (2008).
Brady, R. A., Mocca, C. P., Plaut, R. D., Takeda, K. & Burns, D. L. Comparison of the immune response during acute and chronic Staphylococcus aureus infection. PLoS ONE 13, e0195342 (2018).
Hampton, H. G., Watson, B. N. J. & Fineran, P. C. The arms race between bacteria and their phage foes. Nature 577, 327–336 (2020).
Fothergill, J. L., Neill, D. R., Loman, N., Winstanley, C. & Kadioglu, A. Pseudomonas aeruginosa adaptation in the nasopharyngeal reservoir leads to migration and persistence in the lungs. Nat. Commun. 5, 4780 (2014).
Fux, C. A., Shirtliff, M., Stoodley, P. & Costerton, J. W. Can laboratory reference strains mirror “real-world” pathogenesis? Trends Microbiol. 13, 58–63 (2005).
Palmer, K. L., Aye, L. M. & Whiteley, M. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J. Bacteriol. 189, 8079–8087 (2007).
Turner, K. H., Wessel, A. K., Palmer, G. C., Murray, J. L. & Whiteley, M. Essential genome of Pseudomonas aeruginosa in cystic fibrosis sputum. Proc. Natl Acad. Sci. USA 112, 4110–4115 (2015).
Quickel, K. E. Jr, Selden, R., Caldwell, J. R., Nora, N. F. & Schaffner, W. Efficacy and safety of topical lysostaphin treatment of persistent nasal carriage of Staphylococcus aureus. Appl. Microbiol. 22, 446–450 (1971).
Walsh, S., Shah, A. & Mond, J. Improved pharmacokinetics and reduced antibody reactivity of lysostaphin conjugated to polyethylene glycol. Antimicrob. Agents Chemother. 47, 554–558 (2003).
Kaur, T. et al. Immunocompatibility of bacteriophages as nanomedicines. J. Nanotechnol. 2012, 247427 (2012).
Blazanovic, K. et al. Structure-based redesign of lysostaphin yields potent antistaphylococcal enzymes that evade immune cell surveillance. Mol. Ther. Methods Clin. Dev. 2, 15021 (2015).
Zhao, H. et al. Depletion of T cell epitopes in lysostaphin mitigates anti-drug antibody response and enhances antibacterial efficacy in vivo. Chem. Biol. 22, 629–639 (2015).
Alcantar, N. A., Aydil, E. S. & Israelachvili, J. N. Polyethylene glycol–coated biocompatible surfaces. J. Biomed. Mater. Res. 51, 343–351 (2000).
Zhang, P., Sun, F., Liu, S. & Jiang, S. Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation. J. Control. Rel. 244, 184–193 (2016).
Saifer, M. G. P., Williams, L. D., Sobczyk, M. A., Michaels, S. J. & Sherman, M. R. Selectivity of binding of PEGs and PEG-like oligomers to anti-PEG antibodies induced by methoxyPEG-proteins. Mol. Immunol. 57, 236–246 (2014).
Qi, Y. et al. A brush-polymer/exendin-4 conjugate reduces blood glucose levels for up to five days and eliminates poly(ethylene glycol) antigenicity. Nat. Biomed. Eng. 1, 0002 (2016).
Mancuso, F., Shi, J. & Malik, D. J. High throughput manufacturing of bacteriophages using continuous stirred tank bioreactors connected in series to ensure optimum host bacteria physiology for phage production. Viruses 10, 537 (2018).
Wibowo, D. & Zhao, C.-X. Recent achievements and perspectives for large-scale recombinant production of antimicrobial peptides. Appl. Microbiol. Biotechnol. 103, 659–671 (2019).
Nour El-Din, H. T. et al. A rapid lysostaphin production approach and a convenient novel lysostaphin loaded nano-emulgel; as a sustainable low-cost methicillin-resistant Staphylococcus aureus combating platform. Biomolecules 10, 435 (2020).
Szweda, P., Gorczyca, G., Filipkowski, P., Zalewska, M. & Milewski, S. Efficient production of Staphylococcus simulans lysostaphin in a benchtop bioreactor by recombinant Escherichia coli. Prep. Biochem. Biotechnol. 44, 370–381 (2014).
Mierau, I. et al. Industrial-scale production and purification of a heterologous protein in Lactococcus lactis using the nisin-controlled gene expression system NICE: The case of lysostaphin. Microb. Cell Fact. 4, 15 (2005).
Hu, C. et al. Industrialization of lipid nanoparticles: From laboratory-scale to large-scale production line. Eur. J. Pharm. Biopharm. 109, 206–213 (2016).
Fowler, V. G. Jr et al. Exebacase for patients with Staphylococcus aureus bloodstream infection and endocarditis. J. Clin. Invest. 130, 3750–3760 (2020).
Schuch, R., Nowinski, R. C., Wittekind, M., Lee, H. & Schneider, B. Bacteriophage lysin and antibiotic combinations against gram positive bacteria. US Patent 9889181 (2018).
Stefan, M. New antimicrobial agents. European patent application EP2702070EP2702070 (2014).
Czaplewski, L. et al. Alternatives to antibiotics — a pipeline portfolio review. Lancet Infect. Dis. 16, 239–251 (2016).
Ting, D. S. J., Beuerman, R. W., Dua, H. S., Lakshminarayanan, R. & Mohammed, I. Strategies in translating the therapeutic potentials of host defense peptides. Front. Immunol. 11, 983 (2020).
Abdelkader, K., Gerstmans, H., Saafan, A., Dishisha, T. & Briers, Y. The preclinical and clinical progress of bacteriophages and their lytic enzymes: the parts are easier than the whole. Viruses 11, 96 (2019).
Fridman, O., Goldberg, A., Ronin, I., Shoresh, N. & Balaban, N. Q. Optimization of lag time underlies antibiotic tolerance in evolved bacterial populations. Nature 513, 418–421 (2014).
DiGiandomenico, A. et al. A multifunctional bispecific antibody protects against Pseudomonas aeruginosa. Sci. Transl Med. 6, 262ra155 (2014).
Secher, T. et al. The anti-Pseudomonas aeruginosa antibody Panobacumab is efficacious on acute pneumonia in neutropenic mice and has additive effects with meropenem. PLoS ONE 8, e73396 (2013).
Palmu, A. A. et al. Effect of pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) on outpatient antimicrobial purchases: a double-blind, cluster randomised phase 3–4 trial. Lancet Infect. Dis. 14, 205–212 (2014).
Nuccitelli, A. et al. Structure-based approach to rationally design a chimeric protein for an effective vaccine against Group B Streptococcus infections. Proc. Natl Acad. Sci. USA 108, 10278–10283 (2011).
Hancock, R. E., Nijnik, A. & Philpott, D. J. Modulating immunity as a therapy for bacterial infections. Nat. Rev. Microbiol. 10, 243–254 (2012).
Scott, M. G. et al. An anti-infective peptide that selectively modulates the innate immune response. Nat. Biotechnol. 25, 465–472 (2007).
de la Fuente-Núñez, C., Reffuveille, F., Haney, E. F., Straus, S. K. & Hancock, R. E. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog. 10, e1004152 (2014).
Krausgruber, T. et al. Structural cells are key regulators of organ-specific immune responses. Nature 583, 296–302 (2020).
Todd, E. M., Ramani, R., Szasz, T. P. & Morley, S. C. Inhaled GM-CSF in neonatal mice provides durable protection against bacterial pneumonia. Sci. Adv. 5, eaax3387 (2019).
Zhang, Z., Nong, J. & Zhong, Y. Antibacterial, anti-inflammatory and neuroprotective layer-by-layer coatings for neural implants. J. Neural Eng. 12, 046015 (2015).
Bouras, M., Asehnoune, K. & Roquilly, A. Contribution of dendritic cell responses to sepsis-induced immunosuppression and to susceptibility to secondary pneumonia. Front. Immunol. 9, 2590 (2018).
Hotchkiss, R. S., Monneret, G. & Payen, D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 13, 862–874 (2013).
Roquilly, A. et al. Alveolar macrophages are epigenetically altered after inflammation, leading to long-term lung immunoparalysis. Nat. Immunol. 21, 636–648 (2020).
Lee, J. H., Jeong, S. H., Cha, S.-S. & Lee, S. H. A lack of drugs for antibiotic-resistant Gram-negative bacteria. Nat. Rev. Drug Discov. 6, 938–938 (2007).
York, A. New drugs for the antibacterial pipeline? Nat. Rev. Microbiol. 18, 61–61 (2020).
Jault, P. et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 19, 35–45 (2019).
Leitner, L. et al. Bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: a randomized, placebo-controlled, double-blind clinical trial. BMC Urol. 17, 90 (2017).
Leitner, L. et al. Intravesical bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: a randomised, placebo-controlled, double-blind clinical trial. Lancet Infect. Dis. 21, 427–436 (2020).
Jun, S. Y. et al. Pharmacokinetics and tolerance of the phage endolysin-based candidate drug SAL200 after a single intravenous administration among healthy volunteers. Antimicrob. Agents Chemother. 61, e02629-16 (2017).
Raqib, R. et al. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc. Natl Acad. Sci. USA 103, 9178–9183 (2006).
Raqib, R. et al. Efficacy of sodium butyrate adjunct therapy in shigellosis: a randomized, double-blind, placebo-controlled clinical trial. BMC Infect. Dis. 12, 111 (2012).
Rekha, R. S. et al. Immune responses in the treatment of drug-sensitive pulmonary tuberculosis with phenylbutyrate and vitamin D3 as host directed therapy. BMC Infect. Dis. 18, 303 (2018).
Melo Ld, V. P. et al. Development of a phage cocktail to control proteus mirabilis catheter-associated urinary tract infections. Front. Microbiol. 7, 1024 (2016).
Meyer, A., Greene, M., Kimmelshue, C. & Cademartiri, R. Stabilization of T4 bacteriophage at acidic and basic pH by adsorption on paper. Colloids Surf. B Biointerfaces 160, 169–176 (2017).
Fulgione, A. et al. Biomimetic hydroxyapatite nanocrystals are an active carrier for Salmonella bacteriophages. Int. J. Nanomed. 14, 2219–2232 (2019).
Kłodzińska, S. N. et al. Hyaluronic acid-based nanogels improve in vivo compatibility of the anti-biofilm peptide DJK-5. Nanomedicine 20, 102022 (2019).
Xue, Q. et al. Anti-infective biomaterials with surface-decorated tachyplesin I. Biomaterials 178, 351–362 (2018).
Moosazadeh Moghaddam, M. et al. Comparison of the antibacterial effects of a short cationic peptide and 1% silver bioactive glass against extensively drug-resistant bacteria, Pseudomonas aeruginosa and Acinetobacter baumannii, isolated from burn patients. Amino Acids 50, 1617–1628 (2018).
Chen, H. et al. Versatile antimicrobial peptide-based ZnO quantum dots for in vivo bacteria diagnosis and treatment with high specificity. Biomaterials 53, 532–544 (2015).
Zhang, Y. et al. Antibacterial and biocompatible cross-linked waterborne polyurethanes containing gemini quaternary ammonium salts. Biomacromolecules 19, 279–287 (2018).
Chen, Y. F. et al. Star-shaped polypeptides exhibit potent antibacterial activities. Nanoscale 11, 11696–11708 (2019).
Hesaraki, S., Karimi, M. & Nezafati, N. The synergistic effects of SrF2 nanoparticles, YSZ nanoparticles, and poly-ε-l-lysin on physicomechanical, ion release, and antibacterial-cellular behavior of the flowable dental composites. Mater. Sci. Eng. C 109, 110592 (2020).
Liu, Y. et al. Immunomimetic designer cells protect mice from MRSA infection. Cell 174, 259–270.e11 (2018).
Zhu, C. et al. A hydrogel-based localized release of colistin for antimicrobial treatment of burn wound infection. Macromol. Biosci. 17, 1600320 (2017).
Kuijpers, A. J. et al. In vitro and in vivo evaluation of gelatin-chondroitin sulphate hydrogels for controlled release of antibacterial proteins. Biomaterials 21, 1763–1772 (2000).
Vipra, A. A. et al. Antistaphylococcal activity of bacteriophage derived chimeric protein P128. BMC Microbiol. 12, 41 (2012).
Pangule, R. C. et al. Antistaphylococcal nanocomposite films based on enzyme-nanotube conjugates. ACS Nano 4, 3993–4000 (2010).
Flynn, J., Durack, E., Collins, M. N. & Hudson, S. P. Tuning the strength and swelling of an injectable polysaccharide hydrogel and the subsequent release of a broad spectrum bacteriocin, nisin A. J. Mater. Chem. B 8, 4029–4038 (2020).
Blair, J. M. A., Webber, M. A., Baylay, A. J., Ogbolu, D. O. & Piddock, L. J. V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 13, 42–51 (2015).
Acknowledgements
The authors acknowledge support from the Cystic Fibrosis Foundation (CFF GARCIA17G0) and the National Institutes of Health (R01 AR062920).
Author information
Authors and Affiliations
Contributions
P.P.K. and A.J.G. conceived the idea of the Review, developed the outline, contributed to the literature search, writing, and creation of figures and tables. M.R. contributed to the literature search and writing of the section on bacteriophage-based biomaterials, as well as the creation of figures and tables. All authors contributed to the discussion, review and editing of the entire article content.
Corresponding author
Ethics declarations
Competing interests
A.J.G. is an inventor in a patent application on the lysostaphin-delivering hydrogel filed by the Georgia Tech Research Corporation (no. 16/191,685, filed on 15 November 2018). The authors declare no other competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Therapeutic Proteins Database (THPdb): https://webs.iiitd.edu.in/raghava/thpdb/keyword.php
Rights and permissions
About this article
Cite this article
Kalelkar, P.P., Riddick, M. & García, A.J. Biomaterial-based antimicrobial therapies for the treatment of bacterial infections. Nat Rev Mater 7, 39–54 (2022). https://doi.org/10.1038/s41578-021-00362-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41578-021-00362-4
This article is cited by
-
H2O2 self-supplying and GSH-depleting nanosystem for amplified NIR mediated-chemodynamic therapy of MRSA biofilm-associated infections
Journal of Nanobiotechnology (2024)
-
A biodegradable PVA coating constructed on the surface of the implant for preventing bacterial colonization and biofilm formation
Journal of Orthopaedic Surgery and Research (2024)
-
Prediction of the synergistic effect of antimicrobial peptides and antimicrobial agents via supervised machine learning
BMC Biomedical Engineering (2024)
-
Tetrahedral framework nucleic acids/hyaluronic acid-methacrylic anhydride hybrid hydrogel with antimicrobial and anti-inflammatory properties for infected wound healing
International Journal of Oral Science (2024)
-
Nanobiomaterials: exploring mechanistic roles in combating microbial infections and cancer
Discover Nano (2023)