Abstract
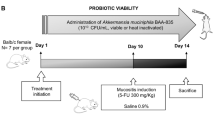
Intestinal mucositis (IM) is a common side effect resulting from cancer treatment. However, the management so far has not been very effective. In the last years, the role of the gut microbiota in the development and severity of mucositis has been studied. Therefore, the use of probiotics and paraprobiotics could have a potential therapeutic effect on IM. The aim of our study was to investigate the impact of the administration of Lacticaseibacillus rhamnosus (L. rhamnosus) CGMCC1.3724 and the paraprobiotic on IM in mice. For 13 days, male Balb/c mice were divided into six groups: control (CTL) and mucositis (MUC)/0.1 mL of saline; CTL LrV and MUC LrV/0.1 mL of 108 CFU of viable Lr; CTL LrI and MUC LrI/0.1 mL of 108 CFU of inactivated Lr. On the 10th day, mice from the MUC, MUC LrV, and MUC LrI groups received an intraperitoneal injection (300 mg/kg) of 5-fluorouracil to induce mucositis. The results showed that the administration of the chemotherapeutic agent increased the weight loss and intestinal permeability of the animals in the MUC and MUC LrV groups. However, administration of paraprobiotic reduced weight loss and maintained PI at physiological levels. The paraprobiotic also preserved the villi and intestinal crypts, reduced the inflammatory infiltrate, and increased the mucus secretion, Muc2 gene expression, and Treg cells frequency.






Similar content being viewed by others
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Vodenkova S, Buchler T, Cervena K, Veskrnova V, Vodicka P, Vymetalkova V (2020) 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol Ther 206:107447. https://doi.org/10.1016/j.pharmthera.2019.107447
Longley DB, Harkin DP, Johnston PG (2003) 5-fluorouracil : mechanisms of action and clinical strategies. Nat Rev Cancer 3:330–338. https://doi.org/10.1038/nrc1074
Li J, Hou N, Faried A, Tsutsumi S, Takeuchi T, Kuwano H (2009) Inhibition of autophagy by 3-MA enhances the effect of 5-FU-induced apoptosis in colon cancer cells. Ann Surg Oncol 16:761–771. https://doi.org/10.1245/s10434-008-0260-0
Sonis ST (2004) The pathobiology of mucositis. Nat Rev Cancer 4(4):277–284. https://doi.org/10.1038/nrc1318
Al-Dasooqi N, Wardill HR, Gibson RJ (2014) Gastrointestinal mucositis: the role of MMP-tight junction interactions in tissue injury. Pathol Oncol Res 20(7):485–491. https://doi.org/10.1007/s12253-013-9733-y
Elting LS, Chang YC (2019) Costs of oral complications of cancer therapies: estimates and a blueprint for future study. JNCI Monographs 2019(53). https://doi.org/10.1093/jncimonographs/lgz010
Lalla RV, Bowen J, Barasch A et al (2014) MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 120(10):1453–1461. https://doi.org/10.1002/cncr.28592
Cinausero M, Aprile G, Ermacora P, Basile D, Vitale MG, Fanotto V, Parisi G, Calvetti L, Sonis ST (2017) New frontiers in the pathobiology and treatment of cancer regimen-related mucosal injury. Front Pharmacol 8:354. https://doi.org/10.3389/fphar.2017.00354
Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, Bruley des Varannes S, Le Vacon F, de La Cochetière MF, (2014) Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis - current evidence and potential clinical applications. Aliment Pharmacol Ther 40(5):409–421. https://doi.org/10.1111/apt.12878
Hamouda N, Sano T, Oikawa Y, Ozaki T, Shimakawa M, Matsumoto K, Amagase K, Higuchi K, Kato S (2017) Apoptosis, dysbiosis and expression of inflammatory cytokines are sequential events in the development of 5-fluorouracil-induced intestinal mucositis in mice. Basic Clin Pharmacol Toxicol 121(3):159–168. https://doi.org/10.1111/bcpt.12793
Li H-L, Lu L, Wang X-S et al (2017) Alteration of gut microbiota and inflammatory cytokine/chemokine profiles in 5-fluorouracil induced intestinal mucositis. Front Cell Infect Microbiol 7:1–14. https://doi.org/10.3389/fcimb.2017.00455
Trindade LM, Martins VD, Rodrigues NM et al (2018) Oral administration of Simbioflora® (synbiotic) attenuates intestinal damage in a mouse model of 5-fluorouracil-induced mucositis. Benef Microbes 9(3):477–486. https://doi.org/10.3920/BM2017.0082
Quaresma M, Damasceno S, Monteiro C et al (2020) Probiotic mixture containing Lactobacillus spp. and Bifidobacterium spp. attenuates 5-fluorouracil-induced intestinal mucositis in mice. Nutr Cancer 72(8):1355–1365. https://doi.org/10.1080/01635581.2019.1675719
Bastos RW, Pedroso SHSP, Vieira AT et al (2016) Saccharomyces cerevisiae UFMG A-905 treatment reduces intestinal damage in a murine model of irinotecan-induced mucositis. Benef Microbes 7(4):549–557. https://doi.org/10.3920/BM2015.0190
Yeung CY, Chan WT, Bin JC, Cheng ML, Liu CY, Chang SW, Chiau JSC, Lee HC (2015) Amelioration of chemotherapy-induced intestinal mucositis by orally administered probiotics in a mouse model. PLoS One 10(9):e0138746. https://doi.org/10.1371/journal.pone.0138746
Koyama S, Fujita H, Shimosato T et al (2018) Septicemia from Lactobacillus rhamnosus GG, from a probiotic enriched yogurt, in a patient with autologous stem cell transplantation. Probiotics Antimicrob Proteins 11(1):295–298. https://doi.org/10.1007/s12602-018-9399-6
Cuevas-González PF, Liceaga AM, Aguilar-Toalá JE (2020) Postbiotics and paraprobiotics: from concepts to applications. Food Res Int 136:109502. https://doi.org/10.1016/j.foodres.2020.109502
De MS, Sichetti M, Muradyan D, Piccioni M, Traina G, Pagiotti R, Pietrella D (2018) Probiotic cell-free supernatants exhibited anti-inflammatory and antioxidant activity on human gut epithelial cells and macrophages stimulated with LPS. Evid Based Complement Alternat Med 2018:1756308. https://doi.org/10.1155/2018/1756308
Piqué N, Berlanga M, Miñana-Galbis D (2019) Health benefits of heat-killed (Tyndallized) probiotics: an overview. Int J Mol Sci 20(10):2534. https://doi.org/10.3390/ijms20102534
Segers ME, Lebeer S (2014) Towards a better understanding of Lactobacillus rhamnosus GG - host interactions. Microb Cell Fact 13(Suppl 1):S7. https://doi.org/10.1186/1475-2859-13-S1-S7
Sanchez M, Darimont C, Drapeau V et al (2014) Effect of Lactobacillus rhamnosus CGMCC.13724 supplementation on weight loss and maintenance in obese men and women. Br J Nutr 111(8):1507–1519. https://doi.org/10.1017/S0007114513003875
Sanchez M, Darimont C, Panahi S, Drapeau V, Marette A, Taylor VH, Doré J, Tremblay A (2017) Effects of a diet-based weight-reducing program with probiotic supplementation on satiety efficiency, eating behaviour traits, and psychosocial behaviours in obese individuals. Nutrients 9(3):284. https://doi.org/10.3390/nu9030284
Resolução Normativa CONCEA (2016) -n-32-de-06.09.2016-D.O.U.-de-08.09.2016-Secao-I-Pag.-05. 2013–2015. https://antigo.mctic.gov.br/mctic/opencms/legislacao/outros_atos/resolucoes/migracao/Resolucao_CONCEA_n_32_de_06092016.html#:~:text=Baixa%20as%20Diretrizes%20de%20Integridad. Accessed 20 Nov 2020
Maioli TU, De Melo SB, Dias MN, Paiva NC, Cardoso VN, Fernandes SO, Carneiro CM, Dos Santos MF, De Vasconcelos GS (2014) Pretreatment with Saccharomyces boulardii does not prevent the experimental mucositis in Swiss mice. J Negat Results Biomed 13:6. https://doi.org/10.1186/1477-5751-13-6
Diniz SO, Resende BM, Nunan EA, Simal CJ, Cardoso VN (1999) 99mTechnetium labelled Escherichia coli. Appl Radiat Isot 51(1):33–36. https://doi.org/10.1016/s0969-8043(98)00185-7
Soares PMG, Mota JMSC, Gomes AS, Oliveira RB, Assreuy AMS, Brito GAC, Santos AA, Ribeiro RA, Souza MHLP (2008) Gastrointestinal dysmotility in 5-fluorouracil-induced intestinal mucositis outlasts inflammatory process resolution. Cancer Chemother Pharmacol 63:91–98. https://doi.org/10.1016/s0969-8043(98)00185-7
Ferreira T, Rasband W (2012) ImageJ User Guide User Guide, IJ 1.46r. https://imagej.nih.gov/ij/docs/guide/user-guide.pdf. Accessed 20 Nov 2020
Jensen EC (2013) Quantitative analysis of histological staining and fluorescence using imageJ. Anat Rec 296(3):378–381. https://doi.org/10.1002/ar.22641
Strath M, Warren DJ, Sanderson CJ (1985) Detection of eosinophils using an eosinophil peroxidase assay. Its use as an assay for eosinophil differentiation factors. J Immunol Methods 83(2):209–215. https://doi.org/10.1016/0022-1759(85)90242-x
Souza DG, Cara DC, Cassali GD, Coutinho SF, Silveira MR, Andrade SP, Poole SP, Teixeira MM (2000) Effects of the PAF receptor antagonist UK74505 on local and remote reperfusion injuries following ischaemia of the superior mesenteric artery in the rat. Br J Pharmacol 131(8):1800–1808. https://doi.org/10.1038/sj.bjp.0703756
Lu Y, Fan C, Li P, Lu Y, Chang X, Qi K (2016) Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating g protein-coupled receptors and gut microbiota. Sci Rep 6:37589. https://doi.org/10.1038/srep37589
Sittipo P, Lobionda S, Lee YK, Maynard CL (2018) Intestinal microbiota and the immune system in metabolic diseases. J Microbiol 56:154–162. https://doi.org/10.1007/s12275-018-7548-y
Bollrath J, Powrie F (2013) Feed your Tregs more fiber. Science 341(6):463–464. https://doi.org/10.1126/science.1242674
Carvalho PLA, Andrade MER, Trindade LM et al (2021) Prophylactic and therapeutic supplementation using fructo-oligosaccharide improves the intestinal homeostasis after mucositis induced by 5- fluorouracil. Biomed Pharmacother 133:111012. https://doi.org/10.1016/j.biopha.2020.111012
De Jesus LCL, Drumond MM, de Carvalho A et al (2019) Protective effect of Lactobacillus delbrueckii subsp. Lactis CIDCA 133 in a model of 5 Fluorouracil-Induced intestinal mucositis. J Funct Foods 53:197–207. https://doi.org/10.1016/j.jff.2018.12.027
de Barros PAV, de Vasconcelos Generoso S, Andrade MER, da Gama MAS, Lopes FCF, de Sales e Souza ÉL, dos Santos Martins F, Miranda SEM, Fernandes SOA, Cardoso VN (2017) Effect of conjugated linoleic acid-enriched butter after 24 hours of intestinal mucositis Induction. Nutr Cancer 69(1):168–175. https://doi.org/10.1080/01635581.2016.1225100
Chang C-W, Liu C-Y, Lee H-C et al (2018) Lactobacillus casei variety rhamnosus probiotic preventively attenuates 5-fluorouracil/oxaliplatin-induced intestinal injury in a syngeneic colorectal cancer model. Front Microbiol 9:983. https://doi.org/10.3389/fmicb.2018.00983
Vinolo MAR, Rodrigues HG, Nachbar RT, Curi R (2011) Regulation of inflammation by short chain fatty acids. Nutrients 3(10):858–876. https://doi.org/10.3390/nu3100858
de Barros PAV, Lacerda Leocádio PC, Alvarez Leite JI et al (2018) Conjugated linoleic acid prevents damage caused by intestinal mucositis induced by 5-fluorouracil in an experimental model. Biomed Pharmacother 103:1567–1576. https://doi.org/10.1016/j.biopha.2018.04.133
Van Spaendonk H, Ceuleers H, Witters L, Patteet E, Joossens J, Augustyns K, Lambeir A-M, De Meester I, De Man JG, De Winter BY (2017) Regulation of intestinal permeability: the role of proteases. World J Gastroenterol 23(12):2106–2123. https://doi.org/10.3748/wjg.v23.i12.2106
Andrade MER, Araújo RS, de Barros PAV, Soares ADN, Abrantes FA, de Generoso S, V, Fernandes SOA, Cardoso VN, (2015) The role of immunomodulators on intestinal barrier homeostasis in experimental models. Clin Nutr 34(6):1080–1087. https://doi.org/10.1016/j.clnu.2015.01.012
Batista VL, da Silva TF, de Jesus LCL, Coelho-Rocha ND, Barroso FAL, Tavares LM, Azevedo V, Mancha-Agresti P, Drumond MM (2020) Probiotics, prebiotics, synbiotics, and paraprobiotics as a therapeutic alternative for intestinal mucositis. Front Microbiol 11:544490. https://doi.org/10.3389/fmicb.2020.544490
Cani PD, Amar J, Iglesias MA et al (2007) Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56(7):1761–1772. https://doi.org/10.2337/db06-1491
Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT (2010) Metabolic syndrome and altered gut microbiota in mice lacking toll-like receptor 5. Science 328(5975):228–231. https://doi.org/10.1126/science.1179721
Li X, Hu D, Tian Y, Song Y, Hou Y, Sun L, Zhang Y, Man C, Zhang W, Jiang Y (2020) Protective effects of a novel Lactobacillus rhamnosus strain with probiotic characteristics against lipopolysaccharide-induced intestinal inflammation in vitro and in vivo. Food Funct 11(7):5799–5814. https://doi.org/10.1039/D0FO00308E
Yan F, Liu L, Dempsey PJ, Tsai YH, Raines EW, Wilson CL, Cao H, Cao Z, Liu L, Polk DB (2013) A Lactobacillus rhamnosus GG-derived soluble protein, p40, stimulates ligand release from intestinal epithelial cells to transactivate epidermal growth factor receptor. J Biol Chem 288(42):30742–30751. https://doi.org/10.1074/jbc.M113.492397
Campos MJ (2011) O papel da imunoglobulina a secretora no mecanismo de defesa da mucosa bucal. Pesqui Bras Odontopediatria Clin Integr 11:139–143
Nishiyama K, Nakamata K, Ueno S, Terao A, Aryantini NPD, Sujaya IN, Fukuda K, Urashima T, Yamamoto Y, Mukai T (2015) Adhesion properties of Lactobacillus rhamnosus mucus-binding factor to mucin and extracellular matrix proteins. Biosci Biotechnol Biochem 79(2):271–279. https://doi.org/10.1080/09168451.2014.972325
Martens EC, Neumann M, Desai MS (2018) Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat Rev Microbiol 16:457–470. https://doi.org/10.1038/s41579-018-0036-x
Balgi AD, Diering GH, Donohue E, Lam KKY, Fonseca BD, Zimmerman C, Numata M, Roberge M (2011) Regulation of mTORC1 signaling by pH. PLoS One 6(6):e21549. https://doi.org/10.1371/journal.pone.0021549
Certo M, Tsai CH, Pucino V, Ho PC, Mauro C (2020) Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat Rev Immunol 21:151–161. https://doi.org/10.1038/s41577-020-0406-2
Kushkevych I, Leščanová O, Dordević D, Jančíková S, Hošek J, Vítězová M, Buňková L, Drago L (2019) The sulfate-reducing microbial communities and meta-analysis of their occurrence during diseases of small–large intestine axis. J Clin Med 8(10):1656. https://doi.org/10.3390/jcm8101656
Pham VT, Lacroix C, Braegger CP, Chassard C (2017) Lactate-utilizing community is associated with gut microbiota dysbiosis in colicky infants. Sci Rep 7(1):11176. https://doi.org/10.1038/s41598-017-11509-1
Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, De Lafaille MAC (2005) Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest 115(7):1923–1933. https://doi.org/10.1172/JCI24487
Coombes JL, Siddiqui KRR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F (2007) A functionally specialized population of mucosal CD103+ DCs induces foxp3+ regulatory T cells via a TGF-β -and retinoic acid-dependent mechanism. J Exp Med 204:1757–1764. https://doi.org/10.1084/jem.20070590
Fujiki T, Hirose Y, Yamamoto Y, Murosaki S (2012) Enhanced immunomodulatory activity and stability in simulated digestive juices of Lactobacillus plantarum L-137 by heat treatment. Biosci Biotechnol Biochem 76(5):918–922. https://doi.org/10.1271/bbb.110919
Grześkowiak L, Collado MC, Beasley S, Salminen S (2014) Pathogen exclusion properties of canine probiotics are influenced by the growth media and physical treatments simulating industrial processes. J Appl Microbiol 116(5):1308–1314. https://doi.org/10.1111/jam.12477
Esterházy D, Canesso MCC, Mesin L, Muller PA, de Castro TBR, Lockhart A, ElJalby M, Faria AMC, Mucida D (2019) Compartmentalized gut lymph node drainage dictates adaptive immune responses. Nature 569:126–130. https://doi.org/10.1038/s41586-019-1125-3
Acknowledgements
The Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES awarded the student’s scholarship.
Funding
This work was supported by grants provided by the Conselho Nacional para o Desenvolvimento Cientifico e Tecnológico from Brazil (CNPq—APQ-00593–14; 303506/2019–9; 428548/2018–0), by the Fundação de Amparo a Pesquisa de Minas Gerais (FAPEMIG—APQ-00593–14; PPM-00083–18) and by the Pro Reitoria de Pesquisa da Universidade Federal de Minas Gerais (PRPq/ UFMG).
Author information
Authors and Affiliations
Contributions
L.M.T, S.V.G., V.N.C., and F.S.M. designed the work; L.M.T., L.T., I.D.M., V.M.S., L.C.L.J., G.C., J.J.S.O., G.C.D., V.A.C.A., and T.U.M. acquired data; P.M.A, L.M.T., S.V.G., and F.S.M. played an important role in interpreting the results; L.M.T, S.V.G., V.N.C., and F.S.M. drafted or revised the manuscript. All authors have read and approved the final manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics Approval
All procedures involving animals were performed according to the guidelines for care and use of laboratory animals indicated by the Conselho Nacional de Experimentação Animal (CONCEA) for the care and use of laboratory animals and approved by the Comissão de Ética no Uso de Animais of Federal University of Minas Gerais, under protocol number 66/2018 and according to ARRIVE guidelines.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Trindade, L.M., Torres, L., Matos, I.D. et al. Paraprobiotic Lacticaseibacillus rhamnosus Protects Intestinal Damage in an Experimental Murine Model of Mucositis. Probiotics & Antimicro. Prot. 15, 338–350 (2023). https://doi.org/10.1007/s12602-021-09842-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-021-09842-z