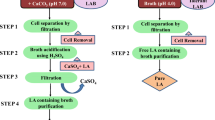
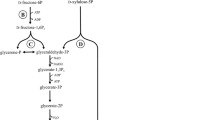
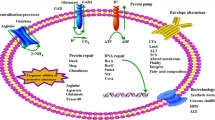
Abstract
Lactic acid can synthesize high value-added chemicals such as poly lactic acid. In order to further minimize the cost of lactic acid production, some effective strategies (e.g., effective mutagenesis and metabolic engineering) have been applied to increase productive capacity of lactic acid bacteria. In addition, low-cost cheap raw materials (e.g., cheap carbon source and cheap nitrogen source) are also used to reduce the cost of lactic acid production. In this review, we summarized the recent developments in lactic acid production, including efficient strain modification technology (high-efficiency mutagenesis means, adaptive laboratory evolution, and metabolic engineering), the use of low-cost cheap raw materials, and also discussed the future prospects of this field, which could promote the development of lactic acid industry.



Similar content being viewed by others
References
Datta, R., & Henry, M. (2006). Lactic acid: Recent advances in products, processes and technologies-a review. Journal of Chemical Technology and Biotechnology, 81(7), 1119–1129.
Gao, C., Ma, C. Q., & Xu, P. (2011). Biotechnological routes based on lactic acid production from biomass. Biotechnology Advances, 29(6), 930–939.
Juturu, V., & Wu, J. C. (2015). Microbial production of lactic acid: The latest development. Critical Reviews in Biotechnology, 36(6), 967–977.
Han, X. S., Han, X., Hong, F., Liu, G., & Bao, J. (2018). An approach of utilizing water-soluble carbohydrates in lignocellulose feedstock for promotion of cellulosic L-lactic acid production. Journal of Agriculture and Food Chemistry, 66(39), 10225–10232.
de Oliveira, A., Komesu, R., Rossell, A. V., Eduardo, C., Filho, M., & Rubens. (2018). Challenges and opportunities in lactic acid bioprocess design from economic to production aspects. Biochemical Engineering Journal, 133, 219–239.
Corma, A., Iborra, S., & Velty, A. (2007). Chemical routes for the transformation of biomass into chemicals. Chemical Reviews, 107(6), 2411–2502.
Singhvi, M., Zendo, T., & Sonomoto, K. (2018). Free lactic acid production under acidic conditions by lactic acid bacteria strains: Challenges and future prospects. Applied Microbiology and Biotechnology, 102(14), 5911–5924.
Alves De Oliveira, R., Alexandri, M., Komesu, A., Venus, J., Vaz Rossell, C. E., & Maciel Filho, R. (2019). Current advances in separation and purification of second-generation lactic acid. Separation and Purification Reviews, 0, 1–17.
Taskin, M., Esim, N., & Ortucu, S. (2012). Efficient production of L-lactic acid from chicken feather protein hydrolysate and sugar beet molasses by the newly isolated Rhizopus oryzae TS-61. Food and Bioproducts Processing, 90(4), 773–779.
Wu, X. F., Jiang, S. T., Liu, M., Pan, L. J., Zheng, Z., & Luo, S. Z. (2011). Production of L-lactic acid by Rhizopus oryzae using semicontinuous fermentation in bioreactor. Journal of Industrial Microbiology and Biotechnology, 38(4), 565–571.
Yu, M. C., Wang, R. C., Wang, C. Y., Duan, K. J., & Sheu, D. C. (2007). Enhanced production of L(+)-lactic acid by floc-form culture of Rhizopus oryzae. Journal of the Chinese Institute of Chemical Engineers, 38(3–4), 223–228.
Gerin, P. A., Dufrence, Y., Bellon-Fontaine, M. N., Asther, M., & Rouxhet, P. G. (1993). Surface properties of the condiospores of Phanerochate chysosporium and their relevance to pellet formation. Journal of Biotechnology, 175, 5135–5144.
Abdullatif, T., & Yang, S. T. (2002). Production of L(+)-lactic acid from glucose and starch by immobilized cells of Rhizopus oryzae in a rotating fibrous bed bioreactor. Biotechnology and Bioengineering, 80(1), 1–12.
Huang, Y. L., Shi, P. X., Wu, Z., Sun, R. S., Kang, S. J., & Zhang, F. (2018). Optimization of L-lactic acid fermentation technology with cassava starch by Rhizopus oryzae. China Brewing, 37(11), 95–101.
Liaud, N., Rosso, M. N., Fabre, N., Crapart, S., Herpoel-Gimbert, I., Sigoillot, J. C., Raouche, S., & Levasseur, A. (2015). L-lactic acid production by Aspergillus brasiliensis overexpressing the heterologous ldha gene from Rhizopus oryzae. Microbial Cell Factories, 14, 66.
Shahri, S. Z., Vahabzadeh, F., & Mogharei, A. (2020). Lactic acid production by loofah-immobilized Rhizopus oryzae through one-step fermentation process using starch substrate. Bioprocess and Biosystems Engineering, 43(2), 333–345.
Abdel-Rahman, M. A., Tashiro, Y., & Sonomoto, K. (2013). Recent advances in lactic acid production by microbial fermentation processes. Biotechnology Advances, 31(6), 877–902.
Porro, D., Brambilla, L., Ranzi, B. M., Martegani, E., & Alberghina, L. (1995). Development of metabolically engineered Saccharomyces cerevisiae cells for the production of lactic acid. Biotechnology Progress, 11(3), 294–298.
Colombié, S., Dequin, S., & Sablayrolles, J. M. (2003). Control of lactate production by Saccharomyces cerevisiae expressing a bacterial LDH gene. Enzyme and Microbial Technology, 33(1), 38–46.
Pacheco, A., Talaia, G., Sa-Pessoa, J., Bessa, D., Goncalves, M. J., Moreira, R., Paiva, S., Casal, M., & Queiros, O. (2012). Lactic acid production in Saccharomyces cerevisiae is modulated by expression of the monocarboxylate transporters Jen1 and Ady2. FEMS Yeast Research, 12(3), 375–381.
Adachi, E., Torigoe, M., Sugiyama, M., Nikawa, J., & Shimizu, K. (1998). Modification of metabolic pathways of Saccharomyces cerevisiae by the expression of lactate dehydrogenase and deletion of pyruvate decarboxylase genes for the lactic acid fermentation at low pH value. Journal of Fermentation and Bioengineering, 86(3), 284–289.
Bianchi, M. M., Protani, F., Liu, C. L., Lievense, J., & Porro, D. (2001). Efficient homolactic fermentation by Kluyveromyces lactis strains defective in pyruvate utilization and transformed with the heterologous LDH gene. Applied and Environment Microbiology, 67(12), 5621–5625.
Vishnu Prasad, J., Sahoo, T. K., Naveen, S., & Jayaraman, G. (2020). Evolutionary engineering of Lactobacillus bulgaricus reduces enzyme usage and enhances conversion of lignocellulosics to D-lactic acid by simultaneous saccharification and fermentation. Biotechnology Biofuels, 13, 171.
Liang, S. X., Jiang, W., Song, Y. B., & Zhou, S. F. (2020). Improvement and metabolomics-based analysis of D-lactic acid production from agro-industrial wastes by Lactobacillus delbrueckii submitted to adaptive laboratory evolution. Journal of Agriculture and Food Chemistry, 68(29), 7660–7669.
Han, X., Huang, K. M., Tang, H. Z., Ni, J., Liu, J. Q., Xu, P., & Tao, F. (2019). Steps to high-performance PLA: economical production of D-lactate enabled by a newly isolated Sporolactobacillus terrae strain. Biotechnology Journal, 14(5), e1800656.
Wang, Y., Huo, K., Gao, L. J., Zhao, G. Q., Wang, B., & Liu, J. L. (2021). Open simultaneous saccharification and fermentation of L-lactic acid by complete utilization of sweet sorghum stalk: A water-saving process. RSC Advances, 11(9), 5284–5290.
Sun, J. D., Wang, Y., Wu, B., Bai, Z. Z., & He, B. F. (2015). Enhanced production of D-lactic acid by Sporolactobacillus sp. Y2–8 mutant generated by atmospheric and room temperature plasma. Biotechnology and Applied Biochemistry, 62(2), 287–292.
Unban, K., Kanpiengjai, A., Takata, G., Uechi, K., Lee, W. C., & Khanongnuch, C. (2017). Amylolytic enzymes acquired from L-lactic acid producing Enterococcus faecium K-1 and improvement of direct lactic acid production from cassava starch. Applied Biochemistry and Biotechnology, 183(1), 155–170.
Senedese, A. L., Maciel Filho, R., & Maciel, M. R. (2015). L-lactic acid production by Lactobacillus rhamnosus ATCC 10863. Scientific World Journal, 2015, 501029.
Jiang, X., Xue, Y. F., Wang, A. Y., Wang, L. M., Zhang, G. M., Zeng, Q. T., Yu, B., & Ma, Y. H. (2013). Efficient production of polymer-grade L-lactate by an alkaliphilic Exiguobacterium sp. strain under nonsterile open fermentation conditions. Bioresource Technology, 143, 665–668.
Reddy, G., Altaf, M. D., Naveena, B. J., Venkateshwar, M., & VijayKumar, E. (2008). Amylolytic bacterial lactic acid fermentation-a review. Biotechnology Advances, 26(1), 22–34.
Singh, S. K., Ahmed, S. U., & Pandey, A. (2006). Metabolic engineering approaches for lactic acid production. Process Biochemistry, 41(5), 991–1000.
Kahrl, F., & Roland-Holst, D. (2008). China’s water-energy nexus. Water Policy, 10, 51–65.
Wang, Y., Meng, H. Y., Cai, D., Wang, B., Qin, P. Y., Wang, Z., & Tan, T. W. (2016). Improvement of L-lactic acid productivity from sweet sorghum juice by repeated batch fermentation coupled with membrane separation. Bioresource Technology, 211, 291–297.
Zhu, Z. M., Zhang, J., Ji, X. M., Fang, Z., Wu, Z. M., Chen, J., & Du, G. C. (2018). Evolutionary engineering of industrial microorganisms-strategies and applications. Applied Microbiology and Biotechnology, 102(11), 4615–4627.
Xiao, H. Q., Lan, L. X., & Li, Y. Z. (2006). Compound mutagenesis of HNO2 and UV of protoplast on screening of enzyme-producing strain. Biotechnology, 16(5), 40–41.
Sun, N., Lee, S., & Song, K. B. (2004). Characterization of a carotenoid-hyperproducing yeast mutant isolated by low-dose gamma irradiation. International Journal of Food Microbiology, 94(3), 263–267.
Xie, M., Zhang, X. L., Hu, X. P., Zhang, Y. J., Peng, D. L., Li, Q., & Li, M. (2018). Mutagenic effects of low-energy N+ ion implantation on the propamocarb-tolerance of nematophagous fungus Lecanicillium attenuatum. Biological Control, 117, 1–5.
Wang, L. Y., Huang, Z. L., Li, G., Zhao, H. X., Xing, X. H., Sun, W. T., Li, H. P., Gou, Z. X., & Bao, C. Y. (2010). Novel mutation breeding method for Streptomyces avermitilis using an atmospheric pressure glow discharge plasma. Journal of Applied Microbiology, 108(3), 851–858.
Ou, P. (2008). Progress in research and application of chemical mutation breeding of industrial microorganisms. J HeZhou University, 24(3), 139–144.
Johnston, A. W. B., Beynon, J. L., Buchanan-Wollaston, A. V., Setchell, S. M., Hirsch, P. R., & Beringer, J. E. (1978). High frequency transfer of nodulating ability between strains and species of Rhizobium. Nature (London), 276, 635–636.
Lotfy, W. A., Ghanem, K. M., & El-Helow, E. R. (2007). Citric acid production by a novel Aspergillus niger isolate: I. Mutagenesis and cost reduction studies. Bioresource Technology, 98(18), 3464–3469.
Ma, Y. B., Wang, Z. Y., Zhu, M., Yu, C. J., Cao, Y. P., Zhang, D. Y., & Zhou, G. K. (2013). Increased lipid productivity and TAG content in Nannochloropsis by heavy-ion irradiation mutagenesis. Bioresource Technology, 136, 360–367.
Chen, S. Q. (2000). Mutation breeding test of Chromogenic bacteria T17-2-39. Jiangsu Food and Fermentation, 2, 9–12.
Zheng, L. L., Sheng, Z. W., Han, B. Y., Tan, L., Li, Y. X., & Guo, G. (2013). Effect of different mutation methods on xylanase production ability by Aspergillus niger. Biotechnology Bulletin, 12, 146–150.
Lv, X. Y., Yu, B., Tian, X. W., Chen, Y., Wang, Z. J., Zhuang, Y. P., & Wang, Y. H. (2016). Effect of pH, glucoamylase, pullulanase and invertase addition on the degradation of residual sugar in L-lactic acid fermentation by Bacillus coagulans HL-5 with corn flour hydrolysate. Journal of the Taiwan Institute of Chemical Engineers, 61, 124–131.
Jiang, A. L., Hu, W., Li, W. J., Liu, L., Tian, X. J., Liu, J., Wang, S. Y., Lu, D., & Chen, J. H. (2018). Enhanced production of L-lactic acid by Lactobacillus thermophilus SRZ50 mutant generated by high-linear energy transfer heavy ion mutagenesis. Engineering in Life Sciences, 18(9), 626–634.
Hua, X. F., Wang, J., Wu, Z. J., Zhang, H. X., Li, H. P., Xing, X. H., & Liu, Z. (2010). A salt tolerant Enterobacter cloacae mutant for bioaugmentation of petroleum- and salt-contaminated soil. Biochemical Engineering Journal, 49(2), 201–206.
Jia, X. J., Peng, X. W., Liu, Y., & Han, Y. J. (2017). Conversion of cellulose and hemicellulose of biomass simultaneously to acetoin by thermophilic simultaneous saccharification and fermentation. Biotechnol Biofuels, 10.
Dragosits, M., & Mattanovich, D. (2013). Adaptive laboratory evolution—Principles and applications for biotechnology. Microbial Cell Factories, 12(1), 64.
Mavrommati, M., Daskalaki, A., Papanikolaou, S., & Aggelis, G. (2021). Adaptive laboratory evolution principles and applications in industrial biotechnology. Biotechnology Advances, 107795.
Sun, X. M., Ren, L. J., Bi, Z. Q., Ji, X. J., Zhao, Q. Y., & Huang, H. (2018). Adaptive evolution of microalgae Schizochytrium sp. under high salinity stress to alleviate oxidative damage and improve lipid biosynthesis. Bioresource Technology, 267, 438–444.
Cubas-Cano, E., González-Fernández, C., & Tomás-Pejó, E. (2019). Evolutionary engineering of Lactobacillus pentosus improves lactic acid productivity from xylose-rich media at low pH. Bioresource Technology, 288, e121540.
Mladenović, D., Pejin, J., Kocić-Tanackov, S., Djukić-Vuković, A., & Mojović, L. (2019). Enhanced lactic acid production by adaptive evolution of Lactobacillus paracasei on agro-industrial substrate. Applied Biochemistry and Biotechnology, 187(3), 753–769.
Nyabako, B. A., Fang, H., Cui, F., Liu, K., Tao, T., Zan, X., & Sun, W. (2020). Enhanced acid tolerance in Lactobacillus acidophilus by atmospheric and room temperature plasma (ARTP) coupled with adaptive laboratory evolution (ALE). Applied Biochemistry and Biotechnology, 191(4), 1499–1514.
Zhang, J., Wu, C. D., Du, G. C., & Chen, J. (2012). Enhanced acid tolerance in Lactobacillus casei by adaptive evolution and compared stress response during acid stress. Biotechnol Bioproc E, 17(2), 283–289.
Upadhyaya, B. P., DeVeaux, L. C., & Christopher, L. P. (2014). Metabolic engineering as a tool for enhanced lactic acid production. Trends in Biotechnology, 32(12), 637–644.
Tian, X. W., Liu, X. H., Zhang, Y. F., Chen, Y., Hang, H. F., Chu, J., & Zhuang, Y. P. (2021). Metabolic engineering coupled with adaptive evolution strategies for the efficient production of high-quality L-lactic acid by Lactobacillus paracasei. Bioresource Technology, 323, e124549.
Yang, X. F., Lai, Z. C., Lai, C. F., Zhu, M. Z., Li, S., Wang, J. F., & Wang, X. N. (2013). Efficient production of L-lactic acid by an engineered Thermoanaerobacterium aotearoense with broad substrate specificity. Biotechnology for Biofuels, 6, 124.
Porro, D., Blanchim, M., Rossella, B., Brambilla, L., Menghini, R., Bolzani, D., Carrera, V., Lievense, J., Liu, C. L., Ranzi, B. M., Frontali, L., & Alberghina, L. (1999). Replacement of a metabolic pathway for large-scale production of lactic acid from engineered yeasts. Applied and Environment Microbiology, 65(9), 4211–4215.
Sybesma, W., Starrenburg, M., Kleerebezem, M., Mierau, I., de Vos, W. M., & Hugenholtz, J. R. (2003). Increased production of folate by metabolic engineering of Lactococcus lactis. Applied and Environment Microbiology, 69(6), 3069–3076.
Koivuranta, K. T., IImen, M., Wiebe, M. G., Ruohonon, L., Suominen, P., & Renttia, M. (2014). L-lactic acid production from D-xylose with Candida sonorensis expressing a heterologous lactate dehydrogenase encoding gene. Microbial Cell Factories, 13.
Dong, X. R., Tian, B., Dai, S., Li, T., Guo, L. N., Tan, Z. F., Jiao, Z., Jin, Q. S., Wang, Y. P., & Hua, Y. J. (2015). Expression of pprI from deinococcus radiodurans improves lactic acid production and stress tolerance in Lactococcus lactis. Plos One, 10(11), e0142918.
Kylä-Nikkilä, K., Hujanen, M., Leisola, M., & Palva, A. (2000). Metabolic engineering of Lactobacillus helveticus CNRZ32 for production of pure L-(+)-lactic acid. Applied and Environmental Microbiology, 66(9), 3835–3841.
Saitoh, S., Ishida, N., Onishi, T., Tokahiro, K., Nagamori, E., Kitamoto, K., & Takahashi, H. (2005). Genetically engineered wine yeast produces a high concentration of L-lactic acid of extremely high optical purity. Applied and Environmental Microbiology, 71(5), 2789–2792.
Wen, J. B., & Bao, J. (2019). Engineering Corynebacterium glutamicum triggers glutamic acid accumulation in biotin-rich corn stover hydrolysate. Biotechnology for Biofuels, 12, 86.
Okano, K., Uematsu, G., Hama, S., Tanaka, T., Noda, H., Kondo, A., & Honda, K. (2018). Metabolic engineering of Lactobacillus plantarum for direct L-lactic acid production from raw corn starch. Biotechnology Journal, 13(5), e1700517.
Qiu, Z. Y., Gao, Q. Q., & Bao, J. (2017). Constructing xylose-assimilating pathways in Pediococcus acidilactici for high titer D-lactic acid fermentation from corn stover feedstock. Bioresource Technology, 245(Pt B), 1369–1376.
Qiu, Z. Y., Gao, Q., & Bao, J. (2018). Engineering Pediococcus acidilactici with xylose assimilation pathway for high titer cellulosic L-lactic acid fermentation. Bioresource Technology, 249, 9–15.
Li, Z., Ding, S. F., Li, Z. P., & Tan, T. W. (2006). L-lactic acid production by Lactobacillus casei fermentation with corn steep liquor-supplemented acid-hydrolysate of soybean meal. Biotechnology Journal, 1(12), 1453–1458.
Olszewska-Widdrat, A., Alexandri, M., Lópe-Gómez, J. P., Schneider, R., & Venus, J. (2020). Batch and continuous lactic acid fermentation based on a multi-substrate approach. Microorganisms, 8(7), 1084.
Lai, F., Jin, Y. L.,Tan, L., He, K. Z., Guo, L., Tian, X. P. Li, J. M. Du, A. P., Huang, Y. H. Zhao, H., & Fang, Y. (2021). Bioconversion of wastewater-derived duckweed to lactic acid through fed-batch fermentation at high-biomass loading. Biomass Conversion and Biorefinery.
Tian, X. J., Hu, W., Chen, J. H., Zhang, W., & Li, W. J. (2020). The supplement of vitamin C facilitates L-lactic acid biosynthesis in Lactobacillus thermophilus A69 from sweet sorghum juice coupled with soybean hydrolysate as feedstocks. Industrial Crops and Products, 146, e112159.
Sun, Y. Q., Liu, H. H., Yang, Y., Zhou, X., & Xiu, Z. L. (2021). High-efficient L-lactic acid production from inedible starchy biomass by one-step open fermentation using thermotolerant Lactobacillus rhamnosus DUT1908. Bioprocess and Biosystems Engineering.
Ouyang, S., Zou, L., Qiao, H., Shi, J., & Ouyang, J. (2020). One-pot process for lactic acid production from wheat straw by an adapted Bacillus coagulans and identification of genes related to hydrolysate-tolerance. Bioresource Technology, 315, e123855.
Hofvendahl, K., & Hahn-Hagerdal, B. (2000). Factors affecting the fermentative lactic acid production from renewable resources. Enzyme and Microbial Technology, 26(2–4), 87–107.
Es, I., Khaneghah, A. M., Barba, F. J., Saraiva, J. A., Sant’Ana, A. S., & BagherHashemi, S. M. B. (2018). Recent advancements in lactic acid production-a review. Food Research International, 107, 763–770.
Grewal, J., & Khare, S. K. (2018). One-pot bioprocess for lactic acid production from lignocellulosic agro-wastes by using ionic liquid stable Lactobacillus brevis. Bioresource Technology, 251, 268–273.
Okano, K., Tanaka, T., Ogino, C., Fukuda, H., & Kondo, A. (2010). Biotechnological production of enantiomeric pure lactic acid from renewable resources: Recent achievements, perspectives, and limits. Applied Microbiology and Biotechnology, 85(3), 413–423.
Anuradha, R., Suresh, A. K., & Venkatesh, K. V. (1999). Simultaneous saccharification and fermentation of starch to lactic acid. Process Biochemistry, 35, 367–375.
Odey, E., Abo, B., Li, Z., & Zhou, X. (2018). Application of lactic acid derived from food waste on pathogen inactivation in fecal sludge: A review on the alternative use of food waste. Environmental Health, 33, 423–431.
Zhou, X., Ye, L., & Wu, J. C. (2013). Efficient production of L-lactic acid by newly isolated thermophilic Bacillus coagulans WCPlO-4 with high glucose tolerance[J]. Applied Microbiology and Biotechnology, 97(10), 4309–4314.
Linko, Y. Y., & Javanainen, P. (1996). Simultaneous liquefaction, saccharification, and lactic acid fermentation on barley starch[J]. Enzyme and Microbial Technology, 19(2), 118–123.
John, R. P., Nampoothiri, K. M., & Pandey, A. (2006). Simultaneous saccharification and L-(+)-lactic acid fermentation of protease-treated wheat bran using mixed culture of Lactobacilli[J]. Biotechnology Letters, 28(22), 1823–1826.
Zhang, D., & Cheryan, M. (1991). Direct fermentation of starch to lactic acid by Lactobacillus amylovorus[J]. Biotechnology Letters, 13(10), 733–738.
FoSon, M. S., & Kwon, Y. J. (2013). Direct fermentation of starch to L-(+)-lactic acid by fed-batch culture of Lactobacillus manihotivorans[J]. Food Science and Biotechnology, 22(1), 289–293.
Fossi, B. T., Tavea, F., Jiwoua, C., & Ndjouenkeu, R. (2011). Simultaneous production of raw starch degrading highly thermostable a-amylase and lactic acid by Lactobacillus fermentation 04BBA19[J]. African Journal of Biotechnology, 10(34), 6564–6574.
Marques, S., Santos, J. A. L., Gírio, F. M., & Roseiro, J. C. (2008). Lactic acid production from recycled paper sludge by simultaneous saccharification and fermentation. Biochemical Engineering Journal, 41, 210–216.
Hu, J., Zhang, Z., Lin, Y., Zhao, S., Mei, Y., Liang, Y., & Peng, N. (2015). High-titer lactic acid production from NaOH-pretreated corn stover by Bacillus coagulans LA204 using fed-batch simultaneous saccharification and fermentation under non-sterile condition. Bioresource Technology, 182, 251–257.
John, R. P., Nampoothiri, K. M., & Pandey, A. (2006). Solid-state fermentation for L-lactic acid production from agro wastes using Lactobacillus delbrueckii. Process Biochemistry, 41(4), 759–763.
Meng, X. Z., & Ragauskas, A. J. (2014). Recent advances in understanding the role of cellulose accessibility in enzymatic hydrolysis of lignocellulosic substrates. Current Opinion in Biotechnology, 27, 150–158.
Shibata, K., Flores, D. M., Kobayashi, G., & Sonomoto, K. (2007). Direct L-lactic acid fermentation with sago starch by a novel amylolytic lactic acid bacterium Enterococcus faecium. Enzyme and Microbial Technology, 41(1–2), 149–155.
Petrov, K., Urshev, Z., & Petrova, P. (2008). L(+)-lactic acid production from starch by a novel amylolytic Lactococcus lactis subsp lactis B84. Food Microbiology, 25(4), 550–557.
Eggleston, G., DeLucca, A., Sklanka, S., Dalley, C., Cyr, E. S., & Powell, R. (2015). Investigation of the stabilization and preservation of sweet sorghum juices. Industrial Crops and Products, 64, 258–270.
Billa, E., Koullas, D. P., Koukios, E. G., & Monties, B. (1997). Structure and composition of sweet sorghum stalk components. Industrial Crops and Products, 6(3–4), 297–302.
Fu, H. M., Chen, Y. H., Yang, X. M., Di, J. Y., Xu, M. G., & Zhang, B. G. (2019). Water resource potential for large-scale sweet sorghum production as bioenergy feedstock in Northern China. Science of the Total Environment, 653, 758–764.
Pal, P., & Dey, P. (2013). Process intensification in lactic acid production by three stage membrane integrated hybrid reactor system. Chemical Engineering and Processing, 64, 1–9.
Pal, P., Sikder, J., Roy, S., & Giorno, L. (2009). Process intensification in lactic acid production: A review of membrane based processes. Chemical Engineering and Processing, 48(11–12), 1549–1559.
Ou, M. S., Awasthi, D., Nieves, I., Wang, L., Erickson, J., Vermerris, W., Ingram, L. O., & Shanmugam, K. T. (2016). Sweet sorghum juice and bagasse as feedstocks for the production of optically pure lactic acid by native and engineered Bacillus coagulans strains. BioEnergy Research, 9(1), 123–131.
Wang, Y., Chang, J. Q., Cai, D., Wang, Z., Qin, P. Y., & Tan, T. W. (2017). Repeated-batch fermentation of L-lactic acid from acid hydrolysate of sweet sorghum juice using mixed neutralizing agent under unsterilized conditions. Journal of Chemical Technology and Biotechnology, 92(7), 1848–1854.
de la Torre, I., Acedos, M. G., Ladero, M., & Santos, V. E. (2019). On the use of resting L. delbrueckii spp. delbrueckii cells for D-lactic acid production from orange peel wastes hydrolysates. Biochemical Engineering Journal, 145, 162–169.
Bai, Z. Z., Gao, Z., Sun, J. F., Wu, B., & He, B. F. (2016). D-Lactic acid production by Sporolactobacillus inulinus YBS1-5 with simultaneous utilization of cottonseed meal and corncob residue. Bioresource Technology, 207, 346–352.
Grewal, J., & Khare, S. K. (2018). One-pot bioprocess for lactic acid production from lignocellulosic agrowastes by using ionic liquid stable Lactobacillus brevis. Bioresource Technology, 251, 268–273.
Ooi, K. Y., & Wu, J. C. (2015). Use of dry yeast cells as a cheap nitrogen source for lactic acid production by thermophilic Bacillus coagulans WCP10-4#. Frontiers of Chemical Science and Engineering, 9(3), 381–385.
Sunhoon Kwon, P. C. L., Lee, E. G., Chang, Y. K., & Chang, N. (2000). Production of lactic acid by Lactobacillus rhamnosus with vitamin-supplemented soybean hydrolysate. Enzyme and Microbial Technology, 26, 209–215.
Yao, W. Y., Xiao, W., Zhu, J., Bo, S., & Miller, C. (2010). Utilization of protein extract from dairy manure as a nitrogen source by Rhizopus oryzae NRRL-395 for L-lactic acid production. Bioresource Technology, 101(11), 4132–4138.
Meng, Y., Xue, Y., Bo, Y., Gao, C., & Ma, Y. H. (2012). Efficient production of L-lactic acid with high optical purity by alkaliphilic Bacillus sp. WL-S20. Bioresource Technology, 116, 334–339.
Tian, X. J., Jiang, A. L., Mao, Y. Q., Wu, B., He, M. X., Hu, W., Chen, J. H., & Li, W. J. (2019). Efficient L-lactic acid production from purified sweet sorghum juice coupled with soybean hydrolysate as nitrogen source by Lactobacillus thermophilus A69 strain. Journal of Chemical Technology and Biotechnology, 94(6), 1752–1759.
Gao, T., Wong, Y. K., Ng, C. K., & Ho, K. P. (2012). L-lactic acid production by Bacillus subtilis MUR1. Bioresource Technology, 121, 105–110.
Gao, M. T., Kaneko, M., Hirata, M., Toorisaka, E., & Hano, T. (2008). Utilization of rice bran as nutrient source for fermentative lactic acid production. Bioresource Technology, 99(9), 3659–3664.
Hu, W., Li, W. J., Yang, H. Q., & Chen, J. H. (2019). Current strategies and future prospects for enhancing microbial production of citric acid. Applied Microbiology and Biotechnology, 103(1), 201–209.
Zhu, X. D., Shi, X., Wang, S. W., Chu, J., Zhu, W. H., Ye, B. C., Zuo, P., & Wang, Y. H. (2019). High-throughput screening of high lactic acid-producing Bacillus coagulans by droplet microfluidic based flow cytometry with fluorescence activated cell sorting. RSC Advances, 9(8), 4507–4513.
Chen, S., Cao, Y., Ferguson, L. R., Shu, Q., & Garg, S. (2012). Flow cytometric assessment of the protectants for enhanced in vitro survival of probiotic lactic acid bacteria through simulated human gastro-intestinal stresses. Applied Microbiology and Biotechnology, 95(2), 345–356.
Patten, C. L., Kirchhof, M. G., Schertzberg, M. R., MortonH, R. A., & Schellhorn, E. (2004). Microarray analysis of RpoS-mediated gene expression in Escherichia coli K-12[J]. Molecular Genetics and Genomics, 272(5), 580–591.
Shiu, S. H., & Borevitz, J. O. (2008). The next generation of microarray research: Applications in evolutionary and ecological genomics[J]. Heredity, 100(2), 141–149.
Luan, G. D., Bao, G. H., Zhao, L., Li, Y., Chen, Z. G., Li, Y., & Cai, Z. (2015). Comparative genome analysis of a thermotolerant Escherichia coli obtained by genome replication engineering assisted continuous evolution(GREACE) and its parent strain provides new understanding of microbial heat tolerance [J]. New Biotechnology, 32(6), 732–738.
Zhu, X., Kong, J. Q., Yang, H., Huang, R., Huang, Y., Yang, D., Shen, B., & Duan, Y. W. (2018). Strain improvement by combined UV mutagenesis and ribosome engineering and subsequent fermentation optimization for enhanced 6’-deoxy-bleomycin Z production[J]. Applied Microbiology and Biotechnology, 102(4), 1651–1661.
Comesu, A., de Oliveira, J. A. R., da Silva Martins, L. H., Wolf Macie, M. R., & Filh, R. M. (2017). Lactic acid production to purification: a review[J]. Bioresources, 12(2), 4364–4383.
Cubas-Cano, E., Venus, J., González-Fernández, C., & Tomás-Pejó, E. (2020). Assessment of different Bacillus coagulans strains for L-lactic acid production from defined media and gardening hydrolysates: Effect of lignocellulosic inhibitors. Journal of Biotechnology, 323, 9–16.
Larsson, S., Reimann, A., Nilvebrant, N. O., & Jonsson, L. J. (1999). Comparison of different methods for the detoxification of lignocellulose hydrolyzates of spruce. Applied Biochemistry and Biotechnology, 77(1–3), 91–103.
Zhao, K., Qiao, Q. G., Chu, D. Q., Gu, H. Q., Dao, T. H., Zhang, J., & Bao, J. (2013). Simultaneous saccharification and high titer lactic acid fermentation of corn stover using a newly isolated lactic acid bacterium Pediococcus acidilactici DQ2. Bioresource Technology, 135, 481–489.
Jin, C., Huang, Z., & Bao, J. (2020). High-titer glutamic acid production from lignocellulose using an engineered Corynebacterium glutamicum with simultaneous co-utilization of xylose and glucose. ACS Sustainable Chemistry & Engineering, 8(16), 6315–6322.
Walton, S. L., Bischoff, K. M., van Heiningen, A. R., & van Walsum, G. P. (2010). Production of lactic acid from hemicellulose extracts by Bacillus coagulans MXL-9. Journal of Industrial Microbiology and Biotechnology, 37(8), 823–830.
Qin, J. Y., Wang, X. W., Zheng, Z. J., Ma, C. Q., Tang, H. Z., & Xu, P. (2010). Production of L-lactic acid by a thermophilic Bacillus mutant using sodium hydroxide as neutralizing agent. Bioresource Technology, 101(19), 7570–7576.
Cheng, K. K., Zhang, J. A., Liu, D. H., Sun, Y., Yang, M. D., & Xu, J. M. (2006). Production of 1,3-propanediol by Klebsiella pneumoniae from glycerol broth. Biotechnology Letters, 28(22), 1817–1821.
Wang, Y., Abdel-Rahman, M. A., Tashiro, Y., Xiao, Y. T., Zendo, T., Sakaic, K., & Sonomoto, K. (2014). L-(+)-Lactic acid production by co-fermentation of cellobiose and xylose without carbon catabolite repression using Enterococcus mundtii QU 25. RSC Advances, 4(42), 22013–22021.
Andrzejewski, B., Eggleston, G., Lingle, S., & Powell, R. (2013). Development of a sweet sorghum juice clarification method in the manufacture of industrial feedstocks for value-added fermentation products. Industrial Crops and Products, 44, 77–87.
Andrzejewski, B., Eggleston, G., & Powell, R. (2013). Pilot plant clarification of sweet sorghum juice and evaporation of raw and clarified juices. Industrial Crops and Products, 49, 648–658.
Acknowledgements
The study was supported financially by the Major Program of the Inner Mongolia Autonomous Region of China (No. 2019ZD021), the National Natural Science Foundation of China Large Science Installation Joint Fund Cultivation Project (No. U1932141), and the National Natural Science Foundation of China Large Science Installation Fund Project (No. U2032210).
Author information
Authors and Affiliations
Contributions
XT: methodology, investigation, writing—original draft. HC: formal analysis, data curation. HL: visualization, resources. JC: funding support, resources, data curation, methodology, writing—review and editing.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
All authors consent to publish the manuscript.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tian, X., Chen, H., Liu, H. et al. Recent Advances in Lactic Acid Production by Lactic Acid Bacteria. Appl Biochem Biotechnol 193, 4151–4171 (2021). https://doi.org/10.1007/s12010-021-03672-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03672-z