Abstract
Purpose
The aim of this study is to explore the effects of liraglutide (LRG) on the expression of FTO, AMPK, and AKT in the visceral adipose tissues of obese and diabetic rats and the underlying mechanisms thereof.
Methods
Thirty SPF-grade, male SD rats were randomly divided into the healthy control, diabetic model (DM), and DM + LRG groups. The DM and DM + LRG groups were administered normal saline and LRG (0.6 mg/kg/d), respectively. After 12 weeks, the body weight of the rats was measured, and their visceral adipose tissues were collected and weighed; the levels of serum biochemical indicators and FTO, AMPK, and AKT in these tissues were then measured using qRT-PCR and western blotting.
Results
Compared to the control group, the body weight and visceral fat accumulation and blood glucose, TG, TC, and LDL-C levels increased significantly, while the HDL-C levels decreased significantly, in the DM group (p < 0.05). After LRG treatment, the HDL-C levels increased significantly, but the levels of the other indicators decreased significantly (p < 0.05). Compared to the control group, the visceral adipose tissue levels of FTO and AKT increased significantly, while the AMPK levels decreased significantly in the DM group (p < 0.05). After LRG treatment, the FTO and AKT levels decreased significantly, and the AMPK levels increased significantly (p < 0.05).
Conclusion
LRG may activate and inhibit the AMPK and AKT pathways, respectively, and decrease FTO expression, thereby alleviating abdominal obesity in type 2 diabetes.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) is a common chronic disease. The prevalence of type 2 diabetes (T2DM) and obesity is rapidly increasing worldwide because of lifestyle changes and accelerated aging, posing a global public health issue [1]. Being overweight and abdominal obesity are the greatest risk factors for T2DM, which aggravate insulin resistance and adversely affect blood glucose control [2]. The fat mass and obesity-associated (FTO) gene, discovered by Frayling et al. is abundantly expressed in adipose tissues [3, 4]. FTO is a transcription co-factor that may affect the process of obesity by modulating the growth, development, and adipogenesis of adipocytes [5]. Accumulating evidence suggests that FTO polymorphisms are closely related to obesity and T2DM [6]. AMP-activated protein kinase (AMPK), a key factor in lipid metabolism, reduces the accumulation of lipids by inhibiting the expression of FTO in skeletal muscle cells [7]. The phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) signaling pathway is a classic pathway affected by the development of T2DM. The activation of PI3K/AKT signaling plays an important role in lipid metabolism and insulin resistance. In endometrial cancer, the expression of FTO is induced by the estrogen-activated pPI3K/AKT pathways in adipocytes [8].
Liraglutide (LRG) is a glucagon-like peptide-1 (GLP-1) receptor agonist (GLP-1RA). Similar to GLP-1, LRG promotes insulin secretion, inhibits glucagon release, and maintains blood glucose stability in a glucose concentration-dependent manner [9]. LRG may also regulate glucose metabolism by improving the constitution of intestinal flora, promoting the enrichment of short-chain fatty acid-producing bacteria (probiotic bacteria, e.g., bifidobacteria). In such a manner, it could alleviate systemic inflammation and elicit a beneficial effect on diabetes [10]. In addition, GLP-1RAs reduce energy intake by delaying gastric emptying, increasing satiety, and suppressing appetite, posing a direct impact on energy balance and body weight control [11]. Long-term combined application of LRG for the treatment of T2DM effectively regulates the level of glycated hemoglobin, markedly reduces the body weight of overweight and abdominally obese T2DM patients, alleviates hyperlipidemia, and reduces the risk of cardiovascular disease [12]. In db/db mice, LRG administration reduces body weight and visceral fat production by activating AMPK and inhibiting AKT [13].
Herein, a streptozotocin (STZ)-induced obese rat model of T2DM was used to observe LRG-induced changes in the visceral adipose tissue expression of FTO, AMPK, and AKT, and how these changes may affect metabolism and visceral fat accumulation. Our study provides an experimental basis for examining the effects of LRG on FTO, AMPK, and AKT expression and suggests a mechanism whereby LRG alleviates abdominal obesity in diabetic rats.
Materials and methods
Animals
SPF-grade male Sprague Dawley (SD) rats, weighing 180 − 220 g, were provided by the Laboratory Animal Center of Xinjiang Medical University (Xinjiang, China). A high-fat and high-sugar diet was provided by Beijing Botai Hongda Biotechnology Co., Ltd (China).
Reagents and instruments
STZ and LRG were purchased from Sigma and Novo Nordisk, Denmark, respectively. Primers for the FTO, AMPK, and AKT genes were obtained from the Beijing Genomics Institute. RNA extraction kits, cDNA reverse transcription kits, and real-time PCR kits were purchased from QIAGEN, Germany. Antibodies against FTO, AMPK, and AKT were purchased from Abcam, USA. A portable blood glucose monitor and test papers were purchased from ACCU-Chek, Germany, and the enzyme-labeled analyzer was obtained from Thermo, USA. The real-time PCR machine, CFX96, and the gel imaging and electrophoresis system were obtained from BIO-RAD, USA.
Establishment of the T2DM model
After adaptive feeding for 1 week, searching the related researches, considering the ethics and modeling rationality of animal experiments, thirty SPF-grade male SD rats were randomly divided into healthy control (n = 10) and diabetic model (DM) (n = 20) groups. Rats in the control group were fed an ordinary diet, while those in the DM group were fed with a high-fat, high-sugar diet for 8 weeks. When the average body weight of DM rats reached 400 − 450 g, streptozotocin was intraperitoneally injected at a dose of 30 mg/kg, and the high-fat, high-sugar diet was continued. Blood was collected from the tail vein of all DM rats after 3 days. Blood glucose ≥ 16.7 mmol/L indicated successful induction of diabetes. Then, the twenty diabetic rats were randomly divided into two subgroups (10 per group). One group received LRG (0.6 mg/kg/d) subcutaneously twice a day, while the other was injected with saline as a control. Meanwhile, the high-fat, high-sugar diet was continued. Accordingly, a total of three groups were created, a healthy control group, a DM + LRG group, and a DM control group. All rats were anesthetized and sacrificed after 12 weeks of LRG or standard saline treatment.
Sample collection
After 12 weeks of treatment, rats were fasted overnight for 12 h and anesthetized by intraperitoneal injection of 3% sodium pentobarbital (40 mg/kg). Blood was collected from the abdominal aorta. The visceral adipose tissues were harvested, weighed, and stored at – 80 °C.
Detection of serum biochemical indicators
Whole blood samples were centrifuged, and the supernatants were collected and stored on ice until analysis. For the detection of the levels of serum glucose, triglycerides, total cholesterol, and high- and low-density lipoproteins, ELISA was performed using specific kits according to the manufacturer’s protocols.
Real-time PCR
Total RNA was extracted from visceral adipose tissue and reverse transcribed into cDNA. The FTO, AMPK, and AKT primer sequences used for real-time RT-PCR are shown in Table 1. The mRNA expression levels of FTO, AMPK, and AKT were determined using qPCR in a total volume of 20 μl with the following parameters: denaturation at 95 °C for 10 min, annealing at 60 °C for 1 min, extension at 95 °C for 15 s, for a total of 40 cycles. β-actin was used as an internal control.
Western blotting
Liquid-frozen visceral adipose tissue was homogenized in lysis buffer containing phosphatase inhibitor and protease, sonicated, and centrifuged at 4 °C for 15 min. The supernatants were collected, and protein concentrations were determined using a BCA protein detection kit. Denatured proteins (20 µg/well) were subjected to 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and then transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was suspended in 5% BSA at ambient temperature for 2 h, left to react with the primary antibodies (FTO, 1:1000; AMPK, 1:2000; AKT, 1:1000; β-actin, 1:2000) and incubated overnight at 4 °C. After washing with TBST thrice, the membrane was incubated with an HRP-labeled secondary antibody (1:10,000) at ambient temperature for 2 h. After washing with TBST thrice, signals were detected by high-sensitivity chemiluminescence (ECL) and quantified using a gel analysis system. β-actin was used as the internal control.
Statistical analysis
Experimental data were analyzed using SPASS 25.0 software. Data were presented as \(\overline{\mathrm{x} }\) ± sd. Differences among the three groups were analyzed using ANOVA, followed by least significant difference t-test(LSD). Results with p values < 0.05 were considered statistically significant.
Results
Body weight and visceral fat accumulation
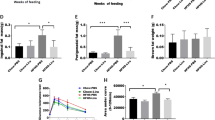
Compared with healthy control rats, the body weight of the DM + LRG and DM rats was significantly increased (p < 0.05) (Table 2, Fig. 1A). However, the body weight of the DM + LRG rats was significantly lower than that of the DM rats (p < 0.05) (Table 2, Fig. 1A). In addition, the weight of visceral fat in DM + LRG and DM rats was significantly higher than that in control rats (p < 0.05) (Table 2, Fig. 1B); however, the visceral fat weight in DM + LRG rats was significantly lower than that in the DM rats (p < 0.05) (Table 2, Fig. 1B).
Glucose and biochemical indicators
Blood glucose (Table 3, Fig. 2A) and the serum triglyceride and total cholesterol (Table 3, Fig. 2B) levels were significantly higher in the DM + LRG and DM rats than in control rats (p < 0.05); however, these levels were significantly lower in DM + LRG rats than in DM rats (p < 0.05) (Table 3, Fig. 2). Moreover, serum high-density lipoprotein cholesterol (HDL-C) levels were significantly lower, while those of low-density lipoprotein cholesterol (LDL-C) were significantly higher in the DM + LRG and DM rats, than in the control rats (p < 0.05) (Table 3, Fig. 2C). However, the DM + LRG rats had significantly higher HDL-C levels, but significantly lower LDL-C levels, than DM rats (p < 0.05) (Table 3, Fig. 2C).
Expression of FTO, AMPK, and AKT in visceral adipose tissues
Compared with control rats, visceral adipocyte FTO and AKT mRNA levels were significantly higher (p < 0.05) (Table 4, Fig. 3A(1) and 3C (1)), while those of AMPK were significantly lower (p < 0.05) (Table 4, Fig. 3B (1)) in DM and DM + LRG rats. However, DM + LRG rats showed significantly lower FTO (Table 4, Fig. 3A (1)) and AKT (Table 4, Fig. 3C (1)) mRNA levels (p < 0.05), and significantly higher AMPK levels (p < 0.05) (Table 4, Fig. 3B (1)) than DM rats.
Similarly, the visceral fat of DM and DM + LRG rats showed significantly higher FTO (Table 5, Fig. 3A (2), Fig. 4) and AKT (Table 5, Fig. 3C(2) and Fig. 4) protein levels, and significantly lower AMPK (Table 5, Fig. 3B(2) and Fig. 4) protein levels. However, DM + LRG rats showed significantly lower FTO (Table 5, Fig. 3A(2) and Fig. 4) and AKT (Table 5, Fig. 3C(2) and Fig. 4) protein levels, but significantly higher AMPK protein levels, than DM rats (p < 0.05) (Table 5, Fig. 3B(2) and Fig. 4).
Discussion
In this study, we used a STZ-induced diabetic obese rat model to observe LRG-induced changes in metabolism and visceral adipose tissue expression of FTO, AMPK, and AKT. Our study provides an experimental basis to study the effects of LRG on FTO, AMPK, and AKT expression and suggests a mechanism whereby LRG may exert its effects in diabetic and obese rats. Many studies have shown that an allele at FTO, rs8050136, is closely related to insulin resistance, inflammatory factors, and obesity markers such as BMI and waist and hip circumference [14,15,16]. Similarly, a polymorphism at FTO rs9939609 is significantly associated with hyperlipidemia, obesity, and insulin metabolism, and this association is mediated by target tissue receptors. Moreover, activation of the SIRT-AMPK signaling pathway reduces the expression of fatty acid synthases and associated transcription factors, increases the oxidation rate of fatty acids, and regulates lipid and energy metabolism [17,18,19]. In a high-fat-induced obesity model in C57BL/6 mice, the activation of AMPK significantly reduced liver fat accumulation and prevented diabetes by inhibiting gluconeogenesis. Insulin-sensitive PI3K/AKT signaling affects glucose and lipid homeostasis in the body [20]. The PI3K/AKT pathway is also involved in the defocused low-energy shock wave in activated adipose tissue-derived stem cells [21]. Inhibition of the PI3K/AKT signaling pathway during liver lipid accumulation promotes hepatocyte autophagy and reduces liver steatosis in db/db mice [22]. In addition, an elevated expression of FTO has been detected in cancer cells, which may regulate cell metabolism and growth via the PI3K/AKT pathway, whereas FTO may be suppressed by the activation of AMPK [23].
Based on the abovementioned findings, the present study explored the expression of FTO, AMPK, and AKT in visceral fat tissue of diabetic obese rats. We found that body weight and visceral fat accumulation were significantly increased in diabetic rats compared to control rats (p < 0.05). In addition, blood glucose, triglyceride, total cholesterol, and LDL-C levels were significantly increased, while those of HDL-C were notably decreased (p < 0.05), in diabetic rats (p < 0.05). The FTO and AKT mRNA and protein levels were significantly elevated, while those of AMPK were decreased, in visceral fat of diabetic rats compared to that of control rats (p < 0.05). These findings suggest that increased FTO and AKT expression, and decreased AMPK levels in visceral fat, may affect fat accumulation in diabetic obese rats. LRG decreases blood sugar levels and may potentially lower apolipoprotein III and triglyceride levels, reducing the levels of lipoprotein particles in atherosclerotic lesions in patients with hyperglycemia [24]. In obese patients, LRG reduces the risk of cardiovascular events by restoring endothelial function [25, 26]. In addition, LRG could alleviate the metabolic status and vascular dysfunction of high-fat-induced obese mice by activating the PKA-AMPK pathway, improving their antioxidative capacity and effecting a protective role within the cardiovascular system [27]. Moreover, inhibition of AKT activity can decrease lipid accumulation [13]. AMPK can promote the dephosphorylation of AKT/PKB via PP2A and thereby affect the activity of AKT in MDA-MB-231 cells [28]. Treatment of diabetic and obese mice with LRG was shown to activate AMPK and inhibit AKT in visceral adipose tissue (composed of perinephric, epididymal, and omental fat), decrease visceral fat accumulation, and reduce body weight [13]. In the present study, we first found that increases in FTO and AKT expression, and reductions in AMPK levels may be involved in the visceral fat accumulation of diabetic and obese rats. Furthermore, we explored the effect of LRG treatment on metabolism and visceral fat tissue expression of FTO, AMPK, and AKT in diabetic and obese rats. We found that treatment with LRG significantly reduced body weight and visceral fat accumulation (p < 0.05); decreased blood glucose, triglyceride, total cholesterol, and LDL-C levels; and increased HDL-C levels in diabetic and obese rats (p < 0.05). Compared with diabetic control rats, the mRNA and protein levels of FTO and AKT were lower, and those of AMPK were higher, in LRG-treated diabetic rats (p < 0.05). These results suggest that LRG may reduce abdominal obesity in type 2 diabetic patients by activating AMPK and suppressing AKT and FTO expression in visceral fat tissue. However, the mechanisms whereby LRG alleviates visceral fat accumulation in type 2 diabetes via the FTO, AMPK, and AKT pathways require further investigation.
In summary, changes in the expression levels of FTO, AMPK, and AKT may affect visceral fat accumulation in diabetic conditions. Mechanically, LRG activates the AMPK pathway, while inhibiting the AKT pathway and decreasing FTO expression, thereby alleviating abdominal obesity in diabetic rats. These findings provide a theoretical basis for the prevention and treatment of type 2 diabetes and abdominal obesity.
References
American Diabetes Association. Prevention or delay of type 2 diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42:S29-33.
Tae JO. The role of anti-obesity medication in prevention of diabetes and its complications. J Obes Metab Syndr. 2019;28:158–66.
Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–94.
Susleyici-Duman B, Zengin K, Kayhan FE, et al. FTO mRNA expression in extremely obese and type 2 diabetic human omental and subcutaneous adipose tissue. Obes Surg. 2011;21:1766–73.
Wu Q, Saunders RA, Szkudlarek-Mikho M, et al. The obesity-associated Fto gene is a transcriptional coactivator. Biochem Biophys Res Commun. 2010;401(3):390–5.
Raza ST, Abbas S, Siddiqi Z, Mahdi F. Association between ACE (rs4646994), FABP2 (rs1799883), MTHFR (rs1801133), FTO (rs9939609) genes polymorphism and type 2 diabetes with dyslipidemia. Int J Mol Cell Med. 2017;6(2):121.
Wu W, Feng J, Jiang D, et al. AMPK regulates lipid accumulation in skeletal muscle cells through FTO-dependent demethylation of N(6)-methyladenosine. Sci Rep. 2017;7:41606.
Zhang Z, Zhou D, Lai Y, et al. Estrogen induces endometrial cancer cell proliferation and invasion by regulating the fat mass and obesity-associated gene via PI3K/AKT and MAPK signaling pathways. Cancer Lett. 2012;319(1):89–97.
Nauck MA, Vardarli I, Deacon CF, et al. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: What is up, what is down? Diabetologia. 2011;54:10–8.
Zhang Q, Xiao XH, Zheng J, et al. Structure moderation of gut microbiota in liraglutide-treated diabetic male rats. Exp Biol Med. 2018;243:34–44.
Van BL, Ijzerman RG, Ten-Kulve JS, et al. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes. 2014;63:4186–96.
Maria M, Eusebio C, Patrizia C, et al. Long-term effectiveness of liraglutide for weight management and glycemic control in type 2 diabetes. Int J Environ Res Public Health. 2020;17:207.
Shao YM, Yuan GH, Zhang JQ, et al. Liraglutide reduces lipogenetic signals in visceral adipose of db/db mice with AMPK activation and Akt suppression. Drug Des Devel Ther. 2015;9:1177–84.
Xiao S, Zeng X, Quan L, et al. Correlation between polymorphism of FTO gene and type 2 diabetes mellitus in Uygur people from northwest China. Int J Clin Exp Med. 2015;8:9744–50.
Amirhosein K, Mehdi KB, Habibesadat S, et al. Association of Omentin rs2274907 and FTO rs9939609 gene polymorphisms with insulin resistance in Iranian individuals with newly diagnosed type 2 diabetes. Lipids Health Dis. 2019;18:142.
Tamer B, Adlija C, Tanja D, et al. Association of FTO gene variant(RS8050136) with type 2 diabetes and markers of obesity, glycaemic, glycaemic control and inflammation. J Med Biochem. 2019;38:153–63.
Huang WC, Peng HL, Hu S, et al. Spilanthol from traditionally used spilanthes acmella enhances AMPK and ameliorates obesity in mice fed high-fat diet. Nutrients. 2019;11:991.
Liou CJ, Lee YK, Ting NC, et al. Protective Effects of Licochalcone A Ameliorates obesity and non-alcoholic fatty liver disease via promotion of the Sirt-1/AMPK pathway in mice fed a high-fat diet. Cells. 2019;8:447.
Tong R, Ang M, Rengong Z, et al. Oleoylethanolamide increases glycogen synthesis and inhibits hepatic gluconeogenesis via the LKB1/AMPK pathway in type 2 diabetic model. J Pharmacol Exp Ther. 2020;373(1):81–91. https://doi.org/10.1124/jpet.119.262675.
Dinda B, Dinda M, Roy A, et al. Dietary plant flavonoids in prevention of obesity and diabetes. Adv Protein Chem Struct Biol. 2020;120:159–235.
Xu L, Zhao Y, Wang M, et al. Defocused low-energy shock wave activates adipose tissue-derived stem cells in vitro via multiple signaling pathways. Cytotherapy. 2016;18(12):1503–14.
Zhong J, Qing Y, Wu SY, et al. Irbesartan alleviates hepatic steatosis in db/db mice by inducing auto-phagy. Chin J Pathophysiol. 2018;34(3):521–7.
Doaei S, Gholamalizadeh M, Akbari ME, et al. Dietary carbohydrate promotes cell survival in cancer via the up-regulation of fat mass and obesity-associated gene expression Level. Malays J Med Sci. 2019;26(2):8–17.
Niina M, Sanni S, Elias BBS, et al. Liraglutide treatment improves postprandial lipid metabolism and cardiometabolic risk factors in humans with adequately controlled type 2 diabetes: a single-centre randomized controlled study. Diabetes Obes Metab. 2019;21:84–94.
Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22.
Rizzo M, Nikolic D, Patti AM, et al. GLP-1 receptor agonists and reduction of cardiometabolic risk: potential underlying mechanisms. Biochim Biophys Acta Mol Basis Dis. 2018;1864:2814–21.
Han F, Hou NN, Liu YP, et al. Liraglutide improves vascular dysfunction by regulating a cAMPindependent PKA-AMPK pathway in perivascular adipose tissue in obese mice. Biomed Pharmacother. 2019;120:109537.
Kim KY, Baek A, Hwang JE, et al. Adiponectin-activated AMPK stimulates dephosphorylation of AKT through protein phosphatase 2A activation. Cancer Res. 2009;69(9):4018–26.
Funding
This work was supported by the State Key Laboratory of Pathogenesis, Prevention, and Treatment of High Incidence Diseases in Central Asia Fund (grant no. SKL-HIDCA-2018–12) and the Xinjiang Uygur Autonomous Region Health and Family Planning Commission young medical science and technology talents special of China (grant no. WJWY-201815).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This study was approved by the Institutional Animal Care and Use Committee (IACUC) of Xinjiang Medical University (Xinjiang, China), and the procedure was strictly performed according to the relevant regulations.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xiao, S., Yang, Y., Liu, Y. et al. Liraglutide may affect visceral fat accumulation in diabetic rats via changes in FTO, AMPK, and AKT expression. Int J Diabetes Dev Ctries 42, 356–362 (2022). https://doi.org/10.1007/s13410-021-00974-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-021-00974-0