Role of Phytochemicals in Cancer Chemoprevention: Insights
Abstract
:1. Introduction
2. Cancer Chemoprevention: Rapidly Growing Field
3. Role of Plant Polyphenols in Chemoprevention of Cancer
4. Natural Chemopreventive Agents in Clinical Setting
4.1. Curcumin
4.2. Resveratrol
4.3. Apigenin
4.4. Epigallocatechin Gallate
4.5. Genistein
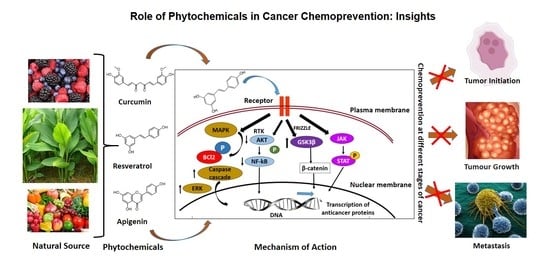
5. Phytochemicals Induced Chemopreventive Mechanisms
5.1. Antiangiogenesis and Metastasis
5.2. Apoptosis and Cell Cycle Arrest
6. Signaling Molecules Involved in Cancer Chemoprevention
6.1. Phytochemicals Modulating Signaling Molecules
6.2. Major Signaling Pathways
7. Role of Antioxidants in Chemoprevention of Cancer
8. Possible Actions of Antioxidant Phytochemicals
9. Role of Natural Products in CYP450 Inhibition
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Flora, S.; Ferguson, L.R. Overview of Mechanisms of Cancer Chemopreventive Agents. Mutat. Res. 2005, 591, 8–15. [Google Scholar] [CrossRef]
- Sporn, M.B. Approaches to Prevention of Epithelial Cancer during the Preneoplastic Period. Cancer Res. 1976, 36, 2699–2702. [Google Scholar]
- Bonovas, S.; Tsantes, A.; Drosos, T.; Sitaras, N.M. Cancer Chemoprevention: A Summary of the Current Evidence. Anticancer Res. 2008, 28, 1857–1866. [Google Scholar]
- Manson, M.M. Cancer Prevention—the Potential for Diet to Modulate Molecular Signalling. Trends Mol. Med. 2003, 9, 11–18. [Google Scholar] [CrossRef]
- Ramos, S. Effects of Dietary Flavonoids on Apoptotic Pathways Related to Cancer Chemoprevention. J. Nutr. Biochem. 2007, 18, 427–442. [Google Scholar] [CrossRef] [Green Version]
- Landis-Piwowar, K.R.; Iyer, N.R. Cancer Chemoprevention: Current State of the Art. Cancer Growth Metastasis 2014, 7, 19–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Vallinas, M.; González-Castejón, M.; Rodríguez-Casado, A.; Ramírez de Molina, A. Dietary Phytochemicals in Cancer Prevention and Therapy: A Complementary Approach with Promising Perspectives. Nutr. Rev. 2013, 71, 585–599. [Google Scholar] [CrossRef]
- Wattenberg, L.W. Chemoprevention of Cancer. Cancer Res. 1985, 45, 1–8. [Google Scholar] [CrossRef]
- Hosseini, A.; Ghorbani, A. Cancer Therapy with Phytochemicals: Evidence from Clinical Studies. Avicenna J. Phytomed. 2015, 5, 84–97. [Google Scholar] [PubMed]
- Sharma, G.; Tyagi, A.K.; Singh, R.P.; Chan, D.C.F.; Agarwal, R. Synergistic Anti-Cancer Effects of Grape Seed Extract and Conventional Cytotoxic Agent Doxorubicin against Human Breast Carcinoma Cells. Breast Cancer Res. Treat. 2004, 85, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lev-Ari, S.; Strier, L.; Kazanov, D.; Madar-Shapiro, L.; Dvory-Sobol, H.; Pinchuk, I.; Marian, B.; Lichtenberg, D.; Arber, N. Celecoxib and Curcumin Synergistically Inhibit the Growth of Colorectal Cancer Cells. Clin. Cancer. Res. 2005, 11, 6738–6744. [Google Scholar] [CrossRef] [Green Version]
- Kabirai, A.; Chahar, A.; Chahar, N.; Gupta, J. Chemical Carcinogenesis: A Brief Review on Mechanism & Metabolism. J. Oral Med. Oral Surg. Oral Pathol. Oral Radiol. 2020, 6, 120–124. [Google Scholar] [CrossRef]
- Malarkey, D.E.; Hoenerhoff, M.; Maronpot, R.R. Chapter 5-Carcinogenesis: Mechanisms and Manifestations. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology, 3rd ed.; Haschek, W.M., Rousseaux, C.G., Wallig, M.A., Eds.; Academic Press: Boston, MA, USA, 2013; pp. 107–146. [Google Scholar] [CrossRef]
- Saha, S.K.; Sikdar, S.; Mukherjee, A.; Bhadra, K.; Boujedaini, N.; Khuda-Bukhsh, A.R. Ethanolic Extract of the Goldenseal, Hydrastis Canadensis, Has Demonstrable Chemopreventive Effects on HeLa Cells in Vitro: Drug-DNA Interaction with Calf Thymus DNA as Target. Environ. Toxicol. Pharmacol. 2013, 36, 202–214. [Google Scholar] [CrossRef]
- Bishayee, K.; Paul, A.; Ghosh, S.; Sikdar, S.; Mukherjee, A.; Biswas, R.; Boujedaini, N.; Khuda-Bukhsh, A.R. Condurango-Glycoside-A Fraction of Gonolobus Condurango Induces DNA Damage Associated Senescence and Apoptosis via ROS-Dependent P53 Signalling Pathway in HeLa Cells. Mol. Cell. Biochem. 2013, 382, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Bishayee, K.; Khuda-Bukhsh, A.R. Graveoline Isolated from Ethanolic Extract of Ruta Graveolens Triggers Apoptosis and Autophagy in Skin Melanoma Cells: A Novel Apoptosis-Independent Autophagic Signaling Pathway. Phytother. Res. 2014, 28, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Sikdar, S.; Mukherjee, A.; Ghosh, S.; Khuda-Bukhsh, A.R. Condurango Glycoside-Rich Components Stimulate DNA Damage-Induced Cell Cycle Arrest and ROS-Mediated Caspase-3 Dependent Apoptosis through Inhibition of Cell-Proliferation in Lung Cancer, in Vitro and in Vivo. Environ. Toxicol. Pharmacol. 2014, 37, 300–314. [Google Scholar] [CrossRef]
- Wang, Z.J.; Chen, X.; Huang, Y.; Lam, W.K.; Lam, W.K.C.; Chow, M.S.S. Overcoming Chemotherapy Resistance with Herbal Medicines: Past, Present and Future Perspectives. Phytochem. Rev. 2014, 13, 323–337. [Google Scholar] [CrossRef]
- Mondal, J.; Bishayee, K.; Panigrahi, A.K.; Khuda-Bukhsh, A.R. Low Doses of Ethanolic Extract of Boldo (Peumus Boldus) Can Ameliorate Toxicity Generated by Cisplatin in Normal Liver Cells of Mice in Vivo and in WRL-68 Cells in Vitro, but Not in Cancer Cells in Vivo or in Vitro. J. Integr. Med. 2014, 12, 425–438. [Google Scholar] [CrossRef]
- Ramos, S. Cancer Chemoprevention and Chemotherapy: Dietary Polyphenols and Signalling Pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [Google Scholar] [CrossRef]
- Arts, I.C.W.; Jacobs, D.R.; Gross, M.; Harnack, L.J.; Folsom, A.R. Dietary Catechins and Cancer Incidence among Postmenopausal Women: The Iowa Women’s Health Study (United States). Cancer Causes Control. 2002, 13, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Key, T.J.; Sharp, G.B.; Appleby, P.N.; Beral, V.; Goodman, M.T.; Soda, M.; Mabuchi, K. Soya Foods and Breast Cancer Risk: A Prospective Study in Hiroshima and Nagasaki, Japan. Br. J. Cancer 1999, 81, 1248–1256. [Google Scholar] [CrossRef] [Green Version]
- Cutler, G.J.; Nettleton, J.A.; Ross, J.A.; Harnack, L.J.; Jacobs, D.R.; Scrafford, C.G.; Barraj, L.M.; Mink, P.J.; Robien, K. Dietary Flavonoid Intake and Risk of Cancer in Postmenopausal Women: The Iowa Women’s Health Study. Int. J. Cancer 2008, 123, 664–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knekt, P.; Järvinen, R.; Seppänen, R.; Hellövaara, M.; Teppo, L.; Pukkala, E.; Aromaa, A. Dietary Flavonoids and the Risk of Lung Cancer and Other Malignant Neoplasms. Am. J. Epidemiol. 1997, 146, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Su, L.J.; Arab, L. Tea Consumption and the Reduced Risk of Colon Cancer—Results from a National Prospective Cohort Study. Public Health Nutr. 2002, 5, 419–425. [Google Scholar] [CrossRef]
- Le Marchand, L.; Murphy, S.P.; Hankin, J.H.; Wilkens, L.R.; Kolonel, L.N. Intake of Flavonoids and Lung Cancer. J. Natl. Cancer Inst. 2000, 92, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Nakachi, K.; Suemasu, K.; Suga, K.; Takeo, T.; Imai, K.; Higashi, Y. Influence of Drinking Green Tea on Breast Cancer Malignancy among Japanese Patients. Jpn. J. Cancer Res. 1998, 89, 254–261. [Google Scholar] [CrossRef]
- Surh, Y.-J. Cancer Chemoprevention with Dietary Phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef]
- Pisters, K.M.; Newman, R.A.; Coldman, B.; Shin, D.M.; Khuri, F.R.; Hong, W.K.; Glisson, B.S.; Lee, J.S. Phase I Trial of Oral Green Tea Extract in Adult Patients with Solid Tumors. J. Clin. Oncol. 2001, 19, 1830–1838. [Google Scholar] [CrossRef]
- Laurie, S.A.; Miller, V.A.; Grant, S.C.; Kris, M.G.; Ng, K.K. Phase I Study of Green Tea Extract in Patients with Advanced Lung Cancer. Cancer Chemother. Pharmacol. 2005, 55, 33–38. [Google Scholar] [CrossRef]
- Jatoi, A.; Ellison, N.; Burch, P.A.; Sloan, J.A.; Dakhil, S.R.; Novotny, P.; Tan, W.; Fitch, T.R.; Rowland, K.M.; Young, C.Y.F.; et al. A Phase II Trial of Green Tea in the Treatment of Patients with Androgen Independent Metastatic Prostate Carcinoma. Cancer 2003, 97, 1442–1446. [Google Scholar] [CrossRef]
- Luo, H.; Tang, L.; Tang, M.; Billam, M.; Huang, T.; Yu, J.; Wei, Z.; Liang, Y.; Wang, K.; Zhang, Z.-Q.; et al. Phase IIa Chemoprevention Trial of Green Tea Polyphenols in High-Risk Individuals of Liver Cancer: Modulation of Urinary Excretion of Green Tea Polyphenols and 8-Hydroxydeoxyguanosine. Carcinogenesis 2006, 27, 262–268. [Google Scholar] [CrossRef] [Green Version]
- Khan, K. Role of Emblica Officinalis in Medicine—A Review. Bot. Res. Int. 2009, 2, 218–228. [Google Scholar]
- Tattelman, E. Health Effects of Garlic. Am. Fam. Physician 2005, 72, 103–106. [Google Scholar]
- Shukla, Y.; Kalra, N. Cancer Chemoprevention with Garlic and Its Constituents. Cancer Lett. 2007, 247, 167–181. [Google Scholar] [CrossRef]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From Ancient Medicine to Current Clinical Trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef]
- Jurenka, J.S. Anti-Inflammatory Properties of Curcumin, a Major Constituent of Curcuma Longa: A Review of Preclinical and Clinical Research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar]
- Akula, A.; Butchi Raju, A.; Prakash, G.; Challa, S. Anti-Cancer Activity of Curcuma Longa Linn. (Turmeric). J. Pharm. Res. 2011, 4, 1274–1276. [Google Scholar]
- Pettit, G.R.; Tan, R.; Ichihara, Y.; Williams, M.D.; Doubek, D.L.; Tackett, L.P.; Schmidt, J.M.; Cerny, R.L.; Boyd, M.R.; Hooper, J.N.A. Antineoplastic Agents, 325. Isolation and Structure of the Human Cancer Cell Growth Inhibitory Cyclic Octapeptides Phakellistatin 10 and 11 from Phakellia Sp. J. Nat. Prod. 1995, 58, 961–965. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Nayak, V.; Vidyasagar, M.S. Evaluation of the Antineoplastic Activity of Guduchi (Tinospora Cordifolia) in Cultured HeLa Cells. Cancer Lett. 1998, 127, 71–82. [Google Scholar] [CrossRef]
- Jada, S.R.; Subur, G.S.; Matthews, C.; Hamzah, A.S.; Lajis, N.H.; Saad, M.S.; Stevens, M.F.G.; Stanslas, J. Semisynthesis and in Vitro Anticancer Activities of Andrographolide Analogues. Phytochemistry 2007, 68, 904–912. [Google Scholar] [CrossRef]
- Sousa, O.; Vieira, G.; Pinho, J.; Yamamoto, C.; Alves, M. Antinociceptive and Anti-Inflammatory Activities of the Ethanol Extract of Annona Muricata L. Leaves in Animal Models. Int. J. Mol. Sci. 2010, 11, 2067–2078. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-T.; Yang, R.-C.; Lee, P.-N.; Yang, S.-H.; Liao, S.-K.; Chen, T.-Y.; Pang, J.-H.S. Anti-Tumor and Anti-Angiogenic Effects of Phyllanthus Urinaria in Mice Bearing Lewis Lung Carcinoma. Int. Immunopharmacol. 2006, 6, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Wall, M.E.; Wani, M.C. Antineoplastic Agents from Plants. Annu. Rev. Pharmacol. Toxicol. 1977, 17, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Malik, F.; Kumar, A.; Bhushan, S.; Khan, S.; Bhatia, A.; Suri, K.A.; Qazi, G.N.; Singh, J. Reactive Oxygen Species Generation and Mitochondrial Dysfunction in the Apoptotic Cell Death of Human Myeloid Leukemia HL-60 Cells by a Dietary Compound Withaferin A with Concomitant Protection by N-Acetyl Cysteine. Apoptosis 2007, 12, 2115–2133. [Google Scholar] [CrossRef]
- Shashi, B.; Jaswant, S.; Madhusudana, R.J.; Kumar, S.A.; Nabi, Q.G. A Novel Lignan Composition from Cedrus Deodara Induces Apoptosis and Early Nitric Oxide Generation in Human Leukemia Molt-4 and HL-60 Cells. Nitric Oxide 2006, 14, 72–88. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Rao, S.K. Evaluation of the Antineoplastic Activity of Guduchi (Tinospora Cordifolia) in Ehrlich Ascites Carcinoma Bearing Mice. Biol. Pharm. Bull. 2006, 29, 460–466. [Google Scholar] [CrossRef] [Green Version]
- George, V.C.; Kumar, D.R.N.; Rajkumar, V.; Suresh, P.K.; Kumar, R.A. Quantitative Assessment of the Relative Antineoplastic Potential of the N-Butanolic Leaf Extract of Annona Muricata Linn. in Normal and Immortalized Human Cell Lines. Asian Pac. J. Cancer Prev. 2012, 13, 699–704. [Google Scholar] [CrossRef]
- Liu, W.; Li, S.-Y.; Huang, X.-E.; Cui, J.-J.; Zhao, T.; Zhang, H. Inhibition of Tumor Growth in Vitro by a Combination of Extracts from Rosa Roxburghii Tratt and Fagopyrum Cymosum. Asian Pac. J. Cancer Prev. 2012, 13, 2409–2414. [Google Scholar] [CrossRef] [Green Version]
- Hematulin, A.; Ingkaninan, K.; Limpeanchob, N.; Sagan, D. Ethanolic Extract from Derris Scandens Benth Mediates Radiosensitzation via Two Distinct Modes of Cell Death in Human Colon Cancer HT-29 Cells. Asian Pac. J. Cancer Prev. 2014, 15, 1871–1877. [Google Scholar] [CrossRef] [Green Version]
- Alabsi, A.M.; Ali, R.; Ali, A.M.; Harun, H.; Al-Dubai, S.A.R.; Ganasegeran, K.; Alshagga, M.A.; Salem, S.D.; Abu Kasim, N.H.B. Induction of Caspase-9, Biochemical Assessment and Morphological Changes Caused by Apoptosis in Cancer Cells Treated with Goniothalamin Extracted from Goniothalamus Macrophyllus. Asian Pac. J. Cancer Prev. 2013, 14, 6273–6280. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Xu, C.-Y.; Cai, S.-Z.; Zhou, Y.; Li, J.; Jiang, R.; Wang, Y.-P. Senescence Effects of Angelica Sinensis Polysaccharides on Human Acute Myelogenous Leukemia Stem and Progenitor Cells. Asian Pac. J. Cancer Prev. 2014, 14, 6549–6556. [Google Scholar] [CrossRef]
- Guo, W.-Q.; Li, L.-Z.; He, Z.-Y.; Zhang, Q.; Liu, J.; Hu, C.-Y.; Qin, F.-J.; Wang, T.-Y. Anti-Proliferative Effects of Atractylis Lancea (Thunb.) DC. via down-Regulation of the c-Myc/HTERT/Telomerase Pathway in Hep-G2 Cells. Asian Pac. J. Cancer Prev. 2013, 14, 6363–6367. [Google Scholar] [CrossRef] [Green Version]
- Cho, W.C.S.; Leung, K.N. In Vitro and in Vivo Anti-Tumor Effects of Astragalus Membranaceus. Cancer Lett. 2007, 252, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Valcic, S.; Timmermann, B.N.; Alberts, D.S.; Wächter, G.A.; Krutzsch, M.; Wymer, J.; Guillén, J.M. Inhibitory Effect of Six Green Tea Catechins and Caffeine on the Growth of Four Selected Human Tumor Cell Lines. Anticancer Drugs 1996, 7, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Parihar, L.; Parihar, P. Review on Cancer and Anticancerous Properties of Some Medicinal Plants. J. Med. Plants Res. 2011, 5, 1818–1835. [Google Scholar]
- Bharali, R.; Azad, M.R.H.; Tabassum, J. Chemopreventive Action of Boerhaavia Diffusa on DMBA-Induced Skin Carcinogenesis in Mice. Indian J. Physiol. Pharmacol. 2003, 47, 459–464. [Google Scholar] [PubMed]
- Patel, S.; Gheewala, N.; Suthar, A.; Shah, A.; Patel, S. In-Vitro Cytotoxicity Activity of Solanum Nigrum Extract against Hela Cell Line and Vero Cell Line. Int. J. Pharm. Pharm. Sci. 2008, 1, 38–46. [Google Scholar]
- Chung, M.J.; Chung, C.-K.; Jeong, Y.; Ham, S.-S. Anticancer Activity of Subfractions Containing Pure Compounds of Chaga Mushroom (Inonotus Obliquus) Extract in Human Cancer Cells and in Balbc/c Mice Bearing Sarcoma-180 Cells. Nutr. Res. Pract. 2010, 4, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, B.S.; Rao, C.V. Chemoprevention of Cancer by Curcumin. In Cancer Chemoprevention: Promising Cancer Chemopreventive Agents; Kelloff, G.J., Hawk, E.T., Sigman, C.C., Eds.; Cancer Drug Discovery and Development; Humana Press: Totowa, NJ, USA, 2004; pp. 169–175. [Google Scholar] [CrossRef]
- Lai, C.-S.; Ho, C.-T.; Pan, M.-H. The Cancer Chemopreventive and Therapeutic Potential of Tetrahydrocurcumin. Biomolecules 2020, 10, 831. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer Potential of Curcumin: Preclinical and Clinical Studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar]
- Prakobwong, S.; Khoontawad, J.; Yongvanit, P.; Pairojkul, C.; Hiraku, Y.; Sithithaworn, P.; Pinlaor, P.; Aggarwal, B.B.; Pinlaor, S. Curcumin Decreases Cholangiocarcinogenesis in Hamsters by Suppressing Inflammation-Mediated Molecular Events Related to Multistep Carcinogenesis. Int. J. Cancer. 2011, 129, 88–100. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Banerjee, S.; Stafford, L.J.; Xia, C.; Liu, M.; Aggarwal, B.B. Curcumin-Induced Suppression of Cell Proliferation Correlates with down-Regulation of Cyclin D1 Expression and CDK4-Mediated Retinoblastoma Protein Phosphorylation. Oncogene 2002, 21, 8852–8861. [Google Scholar] [CrossRef] [Green Version]
- Gururaj, A.E.; Belakavadi, M.; Venkatesh, D.A.; Marmé, D.; Salimath, B.P. Molecular Mechanisms of Anti-Angiogenic Effect of Curcumin. Biochem. Biophys. Res. Commun. 2002, 297, 934–942. [Google Scholar] [CrossRef]
- Hahm, E.-R.; Gho, Y.S.; Park, S.; Park, C.; Kim, K.-W.; Yang, C.-H. Synthetic Curcumin Analogs Inhibit Activator Protein-1 Transcription and Tumor-Induced Angiogenesis. Biochem. Biophys. Res. Commun. 2004, 321, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic Potential of Resveratrol: The In vivo Evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Saiko, P.; Szakmary, A.; Jaeger, W.; Szekeres, T. Resveratrol and Its Analogs: Defense against Cancer, Coronary Disease and Neurodegenerative Maladies or Just a Fad? Mutat. Res. 2008, 658, 68–94. [Google Scholar] [CrossRef] [PubMed]
- Harikumar, K.B.; Aggarwal, B.B. Resveratrol: A Multitargeted Agent for Age-Associated Chronic Diseases. Cell Cycle 2008, 7, 1020–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishayee, A. Cancer Prevention and Treatment with Resveratrol: From Rodent Studies to Clinical Trials. Cancer Prev. Res. 2009, 2, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary Phytochemicals and Cancer Chemoprevention: A Review of the Clinical Evidence. Oncotarget 2016, 7, 52517–52529. [Google Scholar] [CrossRef] [Green Version]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The Therapeutic Potential of Resveratrol: A Review of Clinical Trials. NPJ. Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.L.d.B.; Monteiro, V.V.S.; Navegantes-Lima, K.C.; Reis, J.F.; Gomes, R.d.S.; Rodrigues, D.V.S.; Gaspar, S.L.d.F.; Monteiro, M.C. Resveratrol Role in Autoimmune Disease-A Mini-Review. Nutrients 2017, 9, 1306. [Google Scholar] [CrossRef] [Green Version]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Xue, J.; Li, Y.; Zhang, W.; Ma, D.; Liu, L.; Zhang, Z. Resveratrol Protects against Arsenic Trioxide-Induced Nephrotoxicity by Facilitating Arsenic Metabolism and Decreasing Oxidative Stress. Arch. Toxicol. 2013, 87, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, F.H.; Li, Y.; Wang, Z.; Kong, D. Cellular Signaling Perturbation by Natural Products. Cell. Signal. 2009, 21, 1541–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Xue, J.; Ge, M.; Yu, M.; Liu, L.; Zhang, Z. Resveratrol Attenuates Hepatotoxicity of Rats Exposed to Arsenic Trioxide. Food Chem. Toxicol. 2013, 51, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, G.; Singh, A.K.; Kumar, A.; Prakash, O.; Singh, M.P. Resveratrol Modulates Pyrogallol-Induced Changes in Hepatic Toxicity Markers, Xenobiotic Metabolizing Enzymes and Oxidative Stress. Eur. J. Pharmacol. 2008, 596, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Adhami, V.M.; Ahmad, N. Prevention of Short-Term Ultraviolet B Radiation-Mediated Damages by Resveratrol in SKH-1 Hairless Mice. Toxicol. Appl. Pharmacol. 2003, 186, 28–37. [Google Scholar] [CrossRef]
- Aziz, M.H.; Afaq, F.; Ahmad, N. Prevention of Ultraviolet-B Radiation Damage by Resveratrol in Mouse Skin Is Mediated via Modulation in Survivin. Photochem. Photobiol. 2005, 81, 25–31. [Google Scholar] [CrossRef]
- Aziz, M.H.; Reagan-Shaw, S.; Wu, J.; Longley, B.J.; Ahmad, N. Chemoprevention of Skin Cancer by Grape Constituent Resveratrol: Relevance to Human Disease? FASEB J. 2005, 19, 1193–1195. [Google Scholar] [CrossRef] [Green Version]
- Adhami, V.M.; Afaq, F.; Ahmad, N. Suppression of Ultraviolet B Exposure-Mediated Activation of NF-KappaB in Normal Human Keratinocytes by Resveratrol. Neoplasia 2003, 5, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Reagan-Shaw, S.; Afaq, F.; Aziz, M.H.; Ahmad, N. Modulations of Critical Cell Cycle Regulatory Events during Chemoprevention of Ultraviolet B-Mediated Responses by Resveratrol in SKH-1 Hairless Mouse Skin. Oncogene 2004, 23, 5151–5160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Mao, Y.; Chen, H.; Lin, Y.; Hu, Z.; Wu, J.; Xu, X.; Xu, X.; Qin, J.; Xie, L. Apigenin Promotes Apoptosis, Inhibits Invasion and Induces Cell Cycle Arrest of T24 Human Bladder Cancer Cells. Cancer Cell Int. 2013, 13, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Ning, C.; Wang, Y.; Ma, T.; Huang, H.; Ge, Y.; Liu, J.; Jiang, Y. Natural Plant Flavonoid Apigenin Directly Disrupts Hsp90/Cdc37 Complex and Inhibits Pancreatic Cancer Cell Growth and Migration. J. Funct. Foods 2015, 18, 10–21. [Google Scholar] [CrossRef]
- Salmani, J.M.M.; Zhang, X.-P.; Jacob, J.A.; Chen, B.-A. Apigenin’s Anticancer Properties and Molecular Mechanisms of Action: Recent Advances and Future Prospectives. Chin. J. Nat. Med. 2017, 15, 321–329. [Google Scholar] [CrossRef]
- Yang, J.; Pi, C.; Wang, G. Inhibition of PI3K/Akt/MTOR Pathway by Apigenin Induces Apoptosis and Autophagy in Hepatocellular Carcinoma Cells. Biomed. Pharmacother. 2018, 103, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Afaq, F.; Mukhtar, H. Selective Growth-Inhibitory, Cell-Cycle Deregulatory and Apoptotic Response of Apigenin in Normal versus Human Prostate Carcinoma Cells. Biochem. Biophys. Res. Commun. 2001, 287, 914–920. [Google Scholar] [CrossRef]
- Pham, H.; Chen, M.; Takahashi, H.; King, J.; Reber, H.A.; Hines, O.J.; Pandol, S.; Eibl, G. Apigenin Inhibits NNK-Induced Focal Adhesion Kinase Activation in Pancreatic Cancer Cells. Pancreas 2012, 41, 1306–1315. [Google Scholar] [CrossRef] [Green Version]
- Souza, R.P.; Bonfim-Mendonça, P.d.S.; Gimenes, F.; Ratti, B.A.; Kaplum, V.; Bruschi, M.L.; Nakamura, C.V.; Silva, S.O.; Maria-Engler, S.S.; Consolaro, M.E.L. Oxidative Stress Triggered by Apigenin Induces Apoptosis in a Comprehensive Panel of Human Cervical Cancer-Derived Cell Lines. Oxid. Med. Cell. Longev. 2017, 2017, 1512745. [Google Scholar] [CrossRef]
- Shukla, S.; Bhaskaran, N.; Babcook, M.A.; Fu, P.; Maclennan, G.T.; Gupta, S. Apigenin Inhibits Prostate Cancer Progression in TRAMP Mice via Targeting PI3K/Akt/FoxO Pathway. Carcinogenesis 2014, 35, 452–460. [Google Scholar] [CrossRef] [Green Version]
- Mafuvadze, B.; Liang, Y.; Besch-Williford, C.; Zhang, X.; Hyder, S.M. Apigenin Induces Apoptosis and Blocks Growth of Medroxyprogesterone Acetate-Dependent BT-474 Xenograft Tumors. Horm. Cancer 2012, 3, 160–171. [Google Scholar] [CrossRef] [Green Version]
- Shukla, S.; Fu, P.; Gupta, S. Apigenin Induces Apoptosis by Targeting Inhibitor of Apoptosis Proteins and Ku70-Bax Interaction in Prostate Cancer. Apoptosis 2014, 19, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Kanwal, R.; Shankar, E.; Datt, M.; Chance, M.R.; Fu, P.; MacLennan, G.T.; Gupta, S. Apigenin Blocks IKKα Activation and Suppresses Prostate Cancer Progression. Oncotarget 2015, 6, 31216–31232. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.E.; Young, J.F.; Daneshvar, B.; Lauridsen, S.T.; Knuthsen, P.; Sandström, B.; Dragsted, L.O. Effect of Parsley (Petroselinum Crispum) Intake on Urinary Apigenin Excretion, Blood Antioxidant Enzymes and Biomarkers for Oxidative Stress in Human Subjects. Br. J. Nutr. 1999, 81, 447–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiery-Vuillemin, A.; Nguyen, T.; Pivot, X.; Spano, J.P.; Dufresnne, A.; Soria, J.C. Molecularly Targeted Agents: Their Promise as Cancer Chemopreventive Interventions. Eur. J. Cancer 2005, 41, 2003–2015. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, S.; Cascella, M.; Schiavone, V.; Mehrabi-Kermani, F.; Cuomo, A. The Roles of Epigallocatechin-3-Gallate in the Treatment of Neuropathic Pain: An Update on Preclinical in vivo Studies and Future Perspectives. Drug Des. Devel. Ther. 2017, 11, 2737–2742. [Google Scholar] [CrossRef] [Green Version]
- Gupte, A.; Mumper, R.J. Elevated Copper and Oxidative Stress in Cancer Cells as a Target for Cancer Treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef]
- Khan, H.Y.; Zubair, H.; Ullah, M.F.; Ahmad, A.; Hadi, S.M. Oral Administration of Copper to Rats Leads to Increased Lymphocyte Cellular DNA Degradation by Dietary Polyphenols: Implications for a Cancer Preventive Mechanism. Biometals 2011, 24, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Auger, C.; Mullen, W.; Hara, Y.; Crozier, A. Bioavailability of Polyphenon E Flavan-3-Ols in Humans with an Ileostomy. J. Nutr. 2008, 138, 1535S–1542S. [Google Scholar] [CrossRef] [Green Version]
- Stalmach, A.; Troufflard, S.; Serafini, M.; Crozier, A. Absorption, Metabolism and Excretion of Choladi Green Tea Flavan-3-Ols by Humans. Mol. Nutr. Food Res. 2009, 53, S44–S53. [Google Scholar] [CrossRef]
- Roowi, S.; Stalmach, A.; Mullen, W.; Lean, M.E.J.; Edwards, C.A.; Crozier, A. Green Tea Flavan-3-Ols: Colonic Degradation and Urinary Excretion of Catabolites by Humans. J. Agric. Food Chem. 2010, 58, 1296–1304. [Google Scholar] [CrossRef]
- Mereles, D.; Hunstein, W. Epigallocatechin-3-Gallate (EGCG) for Clinical Trials: More Pitfalls than Promises? Int. J. Mol. Sci. 2011, 12, 5592–5603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedeschi, E.; Suzuki, H.; Menegazzi, M. Antiinflammatory Action of EGCG, the Main Component of Green Tea, through STAT-1 Inhibition. Ann. N. Y. Acad. Sci. 2002, 973, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Dutta, A.; Chatterjee, A. Epigallocatechin-3-Gallate (EGCG) Downregulates Gelatinase-B (MMP-9) by Involvement of FAK/ERK/NFkappaB and AP-1 in the Human Breast Cancer Cell Line MDA-MB-231. Anticancer Drugs 2010, 21, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Wakasaki, T.; Toh, S.; Shimizu, M.; Adachi, S. Chemoprevention of Head and Neck Cancer by Green Tea Extract: EGCG-The Role of EGFR Signaling and “Lipid Raft”. J. Oncol. 2011, 2011, 540148. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, H.; Tighiouart, M.; Lee, J.E.; Shin, H.J.; Khuri, F.R.; Yang, C.S.; Chen, Z.G.; Shin, D.M. Synergistic Inhibition of Head and Neck Tumor Growth by Green Tea (−)-Epigallocatechin-3-Gallate and EGFR Tyrosine Kinase Inhibitor. Int. J. Cancer 2008, 123, 1005–1014. [Google Scholar] [CrossRef] [Green Version]
- Pahlke, G.; Ngiewih, Y.; Kern, M.; Jakobs, S.; Marko, D.; Eisenbrand, G. Impact of Quercetin and EGCG on Key Elements of the Wnt Pathway in Human Colon Carcinoma Cells. J. Agric. Food Chem. 2006, 54, 7075–7082. [Google Scholar] [CrossRef]
- Hossain, M.M.; Banik, N.L.; Ray, S.K. Survivin Knockdown Increased Anti-Cancer Effects of (−)-Epigallocatechin-3-Gallate in Human Malignant Neuroblastoma SK-N-BE2 and SH-SY5Y Cells. Exp. Cell Res. 2012, 318, 1597–1610. [Google Scholar] [CrossRef] [Green Version]
- Vézina, A.; Chokor, R.; Annabi, B. EGCG Targeting Efficacy of NF-ΚB Downstream Gene Products Is Dictated by the Monocytic/Macrophagic Differentiation Status of Promyelocytic Leukemia Cells. Cancer Immunol. Immunother. 2012, 61, 2321–2331. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, M.H.; Jeong, M.; Hwang, Y.S.; Lim, S.H.; Shin, B.A.; Ahn, B.W.; Jung, Y.D. EGCG Blocks Tumor Promoter-Induced MMP-9 Expression via Suppression of MAPK and AP-1 Activation in Human Gastric AGS Cells. Anticancer Res. 2004, 24, 747–753. [Google Scholar]
- Kang, S.U.; Lee, B.-S.; Lee, S.-H.; Baek, S.J.; Shin, Y.S.; Kim, C.-H. Expression of NSAID-Activated Gene-1 by EGCG in Head and Neck Cancer: Involvement of ATM-Dependent P53 Expression. J. Nutr. Biochem. 2013, 24, 986–999. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, N.; Agarwal, C.; Agarwal, R. Differential Responses of Skin Cancer-Chemopreventive Agents Silibinin, Quercetin, and Epigallocatechin 3-Gallate on Mitogenic Signaling and Cell Cycle Regulators in Human Epidermoid Carcinoma A431 Cells. Nutr. Cancer 2001, 39, 292–299. [Google Scholar] [CrossRef]
- Nandakumar, V.; Vaid, M.; Katiyar, S.K. (−)-Epigallocatechin-3-Gallate Reactivates Silenced Tumor Suppressor Genes, Cip1/P21 and P16INK4a, by Reducing DNA Methylation and Increasing Histones Acetylation in Human Skin Cancer Cells. Carcinogenesis 2011, 32, 537–544. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.; Adhami, V.M.; Gupta, S.; Cheng, P.; Mukhtar, H. Role of the Retinoblastoma (PRb)-E2F/DP Pathway in Cancer Chemopreventive Effects of Green Tea Polyphenol Epigallocatechin-3-Gallate. Arch. Biochem. Biophys. 2002, 398, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Erker, L.; Schubert, R.; Yakushiji, H.; Barlow, C.; Larson, D.; Mitchell, J.B.; Wynshaw-Boris, A. Cancer Chemoprevention by the Antioxidant Tempol Acts Partially via the P53 Tumor Suppressor. Hum. Mol. Genet. 2005, 14, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Papadimitrakopoulou, V.A.; Izzo, J.; Mao, L.; Keck, J.; Hamilton, D.; Shin, D.M.; El-Naggar, A.; den Hollander, P.; Liu, D.; Hittelman, W.N.; et al. Cyclin D1 and P16 Alterations in Advanced Premalignant Lesions of the Upper Aerodigestive Tract: Role in Response to Chemoprevention and Cancer Development. Clin. Cancer Res. 2001, 7, 3127–3134. [Google Scholar] [PubMed]
- Tsukamoto, S.; Yamashita, S.; Kim, Y.H.; Kumazoe, M.; Huang, Y.; Yamada, K.; Tachibana, H. Oxygen Partial Pressure Modulates 67-KDa Laminin Receptor Expression, Leading to Altered Activity of the Green Tea Polyphenol, EGCG. FEBS Lett. 2012, 586, 3441–3447. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, Y.; Sumida, M.; Sugihara, K.; Tsukamoto, S.; Yamada, K.; Tachibana, H. Green Tea Polyphenol EGCG Sensing Motif on the 67-KDa Laminin Receptor. PLoS ONE 2012, 7, e37942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.-H.; Kwak, J.; Choi, H.-K.; Choi, K.-C.; Kim, S.; Lee, J.; Jun, W.; Park, H.-J.; Yoon, H.-G. EGCG Suppresses Prostate Cancer Cell Growth Modulating Acetylation of Androgen Receptor by Anti-Histone Acetyltransferase Activity. Int. J. Mol. Med. 2012, 30, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yuan, Y.-Y.; Meeran, S.M.; Tollefsbol, T.O. Synergistic Epigenetic Reactivation of Estrogen Receptor-α (ERα) by Combined Green Tea Polyphenol and Histone Deacetylase Inhibitor in ERα-Negative Breast Cancer Cells. Mol. Cancer 2010, 9, 274. [Google Scholar] [CrossRef] [Green Version]
- Sirtori, C.R.; Arnoldi, A.; Johnson, S.K. Phytoestrogens: End of a Tale? Ann. Med. 2005, 37, 423–438. [Google Scholar] [CrossRef]
- Adjakly, M.; Ngollo, M.; Boiteux, J.-P.; Bignon, Y.-J.; Guy, L.; Bernard-Gallon, D. Genistein and Daidzein: Different Molecular Effects on Prostate Cancer. Anticancer Res. 2013, 33, 39–44. [Google Scholar]
- Dixon, R.A.; Ferreira, D. Genistein. Phytochemistry 2002, 60, 205–211. [Google Scholar] [CrossRef]
- Peterson, G.; Barnes, S. Genistein Inhibition of the Growth of Human Breast Cancer Cells: Independence from Estrogen Receptors and the Multi-Drug Resistance Gene. Biochem. Biophys. Res. Commun. 1991, 179, 661–667. [Google Scholar] [CrossRef]
- Ullrich, A.; Schlessinger, J. Signal Transduction by Receptors with Tyrosine Kinase Activity. Cell 1990, 61, 203–212. [Google Scholar] [CrossRef]
- Okura, A.; Arakawa, H.; Oka, H.; Yoshinari, T.; Monden, Y. Effect of Genistein on Topoisomerase Activity and on the Growth of [Val 12]Ha-Ras-Transformed NIH 3T3 Cells. Biochem. Biophys. Res. Commun. 1988, 157, 183–189. [Google Scholar] [CrossRef]
- Evans, B.A.; Griffiths, K.; Morton, M.S. Inhibition of 5 Alpha-Reductase in Genital Skin Fibroblasts and Prostate Tissue by Dietary Lignans and Isoflavonoids. J. Endocrinol. 1995, 147, 295–302. [Google Scholar] [CrossRef]
- Huang, J.; Nasr, M.; Kim, Y.; Matthews, H.R. Genistein Inhibits Protein Histidine Kinase. J. Biol. Chem. 1992, 267, 15511–15515. [Google Scholar] [CrossRef]
- Zhou, Y.; Lee, A.S. Mechanism for the Suppression of the Mammalian Stress Response by Genistein, an Anticancer Phytoestrogen from Soy. J. Natl. Cancer Inst. 1998, 90, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt Promotes Cell Survival by Phosphorylating and Inhibiting a Forkhead Transcription Factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef] [Green Version]
- Park, W.; Amin, A.R.M.R.; Chen, Z.G.; Shin, D.M. New Perspectives of Curcumin in Cancer Prevention. Cancer Prev. Res. 2013, 6, 387–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, B.; Chung, H.Y.; Kim, N.D. Role of Apigenin in Cancer Prevention via the Induction of Apoptosis and Autophagy. J. Cancer Prev. 2016, 21, 216–226. [Google Scholar] [CrossRef] [Green Version]
- Schramm, L. Going Green: The Role of the Green Tea Component EGCG in Chemoprevention. J. Carcinog. Mutagen. 2013, 4, 1000142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavese, J.M.; Farmer, R.L.; Bergan, R.C. Inhibition of Cancer Cell Invasion and Metastasis by Genistein. Cancer Metastasis Rev. 2010, 29, 465–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annabi, B.; Lachambre, M.-P.; Bousquet-Gagnon, N.; Pagé, M.; Gingras, D.; Béliveau, R. Green Tea Polyphenol (−)-Epigallocatechin 3-Gallate Inhibits MMP-2 Secretion and MT1-MMP-Driven Migration in Glioblastoma Cells. Biochim. Biophys. Acta (BBA)–Mol. Cell Res. 2002, 1542, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Bagchi, D.; Sen, C.K.; Bagchi, M.; Atalay, M. Anti-Angiogenic, Antioxidant, and Anti-Carcinogenic Properties of a Novel Anthocyanin-Rich Berry Extract Formula. Biochemistry 2004, 69, 75–80. [Google Scholar] [CrossRef]
- Yamakawa, S.; Asai, T.; Uchida, T.; Matsukawa, M.; Akizawa, T.; Oku, N. (−)-Epigallocatechin Gallate Inhibits Membrane-Type 1 Matrix Metalloproteinase, MT1-MMP, and Tumor Angiogenesis. Cancer Lett. 2004, 210, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Labrecque, L.; Lamy, S.; Chapus, A.; Mihoubi, S.; Durocher, Y.; Cass, B.; Bojanowski, M.W.; Gingras, D.; Béliveau, R. Combined Inhibition of PDGF and VEGF Receptors by Ellagic Acid, a Dietary-Derived Phenolic Compound. Carcinogenesis 2005, 26, 821–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, R.; Kim, M.H. Epigallocatechin Gallate Inhibits HIF-1alpha Degradation in Prostate Cancer Cells. Biochem. Biophys. Res. Commun. 2005, 334, 543–548. [Google Scholar] [CrossRef]
- Kim, J.-D.; Liu, L.; Guo, W.; Meydani, M. Chemical Structure of Flavonols in Relation to Modulation of Angiogenesis and Immune-Endothelial Cell Adhesion. J. Nutr. Biochem. 2006, 17, 165–176. [Google Scholar] [CrossRef]
- Schindler, R.; Mentlein, R. Flavonoids and Vitamin E Reduce the Release of the Angiogenic Peptide Vascular Endothelial Growth Factor from Human Tumor Cells. J. Nutr. 2006, 136, 1477–1482. [Google Scholar] [CrossRef] [Green Version]
- Woodhouse, E.C.; Chuaqui, R.F.; Liotta, L.A. General Mechanisms of Metastasis. Cancer 1997, 80, 1529–1537. [Google Scholar] [CrossRef]
- Babich, H.; Pinsky, S.M.; Muskin, E.T.; Zuckerbraun, H.L. In vitro Cytotoxicity of a Theaflavin Mixture from Black Tea to Malignant, Immortalized, and Normal Cells from the Human Oral Cavity. Toxicol. In Vitro 2006, 20, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.K.; Koponen, J.M.; Mykkänen, H.M.; Törrönen, A.R. Berry Phenolic Extracts Modulate the Expression of P21(WAF1) and Bax but Not Bcl-2 in HT-29 Colon Cancer Cells. J. Agric. Food Chem. 2007, 55, 1156–1163. [Google Scholar] [CrossRef]
- Feng, R.; Ni, H.-M.; Wang, S.Y.; Tourkova, I.L.; Shurin, M.R.; Harada, H.; Yin, X.-M. Cyanidin-3-Rutinoside, a Natural Polyphenol Antioxidant, Selectively Kills Leukemic Cells by Induction of Oxidative Stress. J. Biol. Chem. 2007, 282, 13468–13476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manna, S.; Banerjee, S.; Mukherjee, S.; Das, S.; Panda, C.K. Epigallocatechin Gallate Induced Apoptosis in Sarcoma180 Cells In vivo: Mediated by P53 Pathway and Inhibition in U1B, U4-U6 UsnRNAs Expression. Apoptosis 2006, 11, 2267–2276. [Google Scholar] [CrossRef]
- Hastak, K.; Agarwal, M.K.; Mukhtar, H.; Agarwal, M.L. Ablation of Either P21 or Bax Prevents P53-Dependent Apoptosis Induced by Green Tea Polyphenol Epigallocatechin-3-Gallate. FASEB J. 2005, 19, 789–791. [Google Scholar] [CrossRef]
- Kundu, T.; Dey, S.; Roy, M.; Siddiqi, M.; Bhattacharya, R.K. Induction of Apoptosis in Human Leukemia Cells by Black Tea and Its Polyphenol Theaflavin. Cancer Lett. 2005, 230, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Kalra, N.; Seth, K.; Prasad, S.; Singh, M.; Pant, A.B.; Shukla, Y. Theaflavins Induced Apoptosis of LNCaP Cells Is Mediated through Induction of P53, down-Regulation of NF-Kappa B and Mitogen-Activated Protein Kinases Pathways. Life Sci. 2007, 80, 2137–2146. [Google Scholar] [CrossRef]
- Favot, L.; Martin, S.; Keravis, T.; Andriantsitohaina, R.; Lugnier, C. Involvement of Cyclin-Dependent Pathway in the Inhibitory Effect of Delphinidin on Angiogenesis. Cardiovasc. Res. 2003, 59, 479–487. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.; Favot, L.; Matz, R.; Lugnier, C.; Andriantsitohaina, R. Delphinidin Inhibits Endothelial Cell Proliferation and Cell Cycle Progression through a Transient Activation of ERK-1/-2. Biochem. Pharmacol. 2003, 65, 669–675. [Google Scholar] [CrossRef]
- Pérez-Ruiz, E.; Melero, I.; Kopecka, J.; Sarmento-Ribeiro, A.B.; García-Aranda, M.; De Las Rivas, J. Cancer Immunotherapy Resistance Based on Immune Checkpoints Inhibitors: Targets, Biomarkers, and Remedies. Drug Resist. Updates 2020, 53, 100718. [Google Scholar] [CrossRef]
- Farooqi, A.A.; de la Roche, M.; Djamgoz, M.B.A.; Siddik, Z.H. Overview of the Oncogenic Signaling Pathways in Colorectal Cancer: Mechanistic Insights. Semin. Cancer Biol. 2019, 58, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Gasparri, M.L.; Bardhi, E.; Ruscito, I.; Papadia, A.; Farooqi, A.A.; Marchetti, C.; Bogani, G.; Ceccacci, I.; Mueller, M.D.; Benedetti Panici, P. PI3K/AKT/MTOR Pathway in Ovarian Cancer Treatment: Are We on the Right Track? Geburtshilfe Frauenheilkd 2017, 77, 1095–1103. [Google Scholar] [CrossRef] [Green Version]
- Patterson, S.L.; Colbert Maresso, K.; Hawk, E. Cancer Chemoprevention: Successes and Failures. Clin. Chem. 2013, 59, 94–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, R.; Kong, A.-N.T. Activation of MAP Kinases, Apoptosis and Nutrigenomics of Gene Expression Elicited by Dietary Cancer-Prevention Compounds. Nutrition 2004, 20, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Kong, A.-N.T. Dietary Cancer-Chemopreventive Compounds: From Signaling and Gene Expression to Pharmacological Effects. Trends Pharmacol. Sci. 2005, 26, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Michl, C.; Vivarelli, F.; Weigl, J.; Nicola, G.R.D.; Canistro, D.; Paolini, M.; Iori, R.; Rascle, A. The Chemopreventive Phytochemical Moringin Isolated from Moringa Oleifera Seeds Inhibits JAK/STAT Signaling. PLoS ONE 2016, 11, e0157430. [Google Scholar] [CrossRef]
- Cohen, S.M.; Arnold, L.L. Chemical Carcinogenesis. Toxicol. Sci. 2011, 120, S76–S92. [Google Scholar] [CrossRef] [PubMed]
- Burotto, M.; Chiou, V.L.; Lee, J.-M.; Kohn, E.C. The MAPK Pathway across Different Malignancies: A New Perspective. Cancer 2014, 120, 3446–3456. [Google Scholar] [CrossRef] [Green Version]
- Adachi, S.; Shimizu, M.; Shirakami, Y.; Yamauchi, J.; Natsume, H.; Matsushima-Nishiwaki, R.; To, S.; Weinstein, I.B.; Moriwaki, H.; Kozawa, O. (−)-Epigallocatechin Gallate Downregulates EGF Receptor via Phosphorylation at Ser1046/1047 by P38 MAPK in Colon Cancer Cells. Carcinogenesis 2009, 30, 1544–1552. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-H.; Jan, R.-L.; Kuo, C.-H.; Chu, Y.-T.; Wang, W.-L.; Lee, M.-S.; Chen, H.-N.; Hung, C.-H. Natural Flavone Kaempferol Suppresses Chemokines Expression in Human Monocyte THP-1 Cells through MAPK Pathways. J. Food Sci. 2010, 75, H254–H259. [Google Scholar] [CrossRef] [PubMed]
- Karar, J.; Maity, A. PI3K/AKT/MTOR Pathway in Angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.M.; Jin, K.-S.; Lee, Y.-W.; Song, Y.S. Luteolin and Chicoric Acid Synergistically Inhibited Inflammatory Responses via Inactivation of PI3K-Akt Pathway and Impairment of NF-ΚB Translocation in LPS Stimulated RAW 264.7 Cells. Eur. J. Pharmacol. 2011, 660, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Zanotto-Filho, A.; Braganhol, E.; Edelweiss, M.I.; Behr, G.A.; Zanin, R.; Schröder, R.; Simões-Pires, A.; Battastini, A.M.O.; Moreira, J.C.F. The Curry Spice Curcumin Selectively Inhibits Cancer Cells Growth In vitro and in Preclinical Model of Glioblastoma. J. Nutr. Biochem. 2012, 23, 591–601. [Google Scholar] [CrossRef]
- Sun, Z.-J.; Chen, G.; Hu, X.; Zhang, W.; Liu, Y.; Zhu, L.-X.; Zhou, Q.; Zhao, Y.-F. Activation of PI3K/Akt/IKK-Alpha/NF-KappaB Signaling Pathway Is Required for the Apoptosis-Evasion in Human Salivary Adenoid Cystic Carcinoma: Its Inhibition by Quercetin. Apoptosis 2010, 15, 850–863. [Google Scholar] [CrossRef]
- Prasad, R.; Vaid, M.; Katiyar, S.K. Grape Proanthocyanidin Inhibit Pancreatic Cancer Cell Growth In vitro and In vivo through Induction of Apoptosis and by Targeting the PI3K/Akt Pathway. PLoS ONE 2012, 7, e43064. [Google Scholar] [CrossRef]
- Shin, D.Y.; Kim, G.-Y.; Lee, J.H.; Choi, B.T.; Yoo, Y.H.; Choi, Y.H. Apoptosis Induction of Human Prostate Carcinoma DU145 Cells by Diallyl Disulfide via Modulation of JNK and PI3K/AKT Signaling Pathways. Int. J. Mol. Sci. 2012, 13, 14158–14171. [Google Scholar] [CrossRef]
- Pawlik, A.; Wiczk, A.; Kaczyńska, A.; Antosiewicz, J.; Herman-Antosiewicz, A. Sulforaphane Inhibits Growth of Phenotypically Different Breast Cancer Cells. Eur. J. Nutr. 2013, 52, 1949–1958. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-H.; Chang, C.-Y.; Lee, K.-R.; Lin, H.-J.; Chen, T.-H.; Wan, L. Flavones Inhibit Breast Cancer Proliferation through the Akt/FOXO3a Signaling Pathway. BMC Cancer 2015, 15, 958. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 Signalling in Cancer: New and Unexpected Biological Functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef]
- Kim, D.-H.; Park, K.-W.; Chae, I.G.; Kundu, J.; Kim, E.-H.; Kundu, J.K.; Chun, K.-S. Carnosic Acid Inhibits STAT3 Signaling and Induces Apoptosis through Generation of ROS in Human Colon Cancer HCT116 Cells. Mol. Carcinog. 2016, 55, 1096–1110. [Google Scholar] [CrossRef]
- Wang, D.; Wise, M.L.; Li, F.; Dey, M. Phytochemicals Attenuating Aberrant Activation of β-Catenin in Cancer Cells. PLoS ONE 2012, 7, e50508. [Google Scholar] [CrossRef] [Green Version]
- Taipale, J.; Beachy, P.A. The Hedgehog and Wnt Signalling Pathways in Cancer. Nature 2001, 411, 349–354. [Google Scholar] [CrossRef]
- Driehuis, E.; Clevers, H. WNT Signalling Events near the Cell Membrane and Their Pharmacological Targeting for the Treatment of Cancer. Br. J. Pharmacol. 2017, 174, 4547–4563. [Google Scholar] [CrossRef] [Green Version]
- Tsai, J.-H.; Hsu, L.-S.; Lin, C.-L.; Hong, H.-M.; Pan, M.-H.; Way, T.-D.; Chen, W.-J. 3,5,4′-Trimethoxystilbene, a Natural Methoxylated Analog of Resveratrol, Inhibits Breast Cancer Cell Invasiveness by Downregulation of PI3K/Akt and Wnt/β-Catenin Signaling Cascades and Reversal of Epithelial-Mesenchymal Transition. Toxicol. Appl. Pharmacol. 2013, 272, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Gwak, J.; Park, S.; Yang, C.S. Green Tea Polyphenol EGCG Suppresses Wnt/β-Catenin Signaling by Promoting GSK-3β- and PP2A-Independent β-Catenin Phosphorylation/Degradation. Biofactors 2014, 40, 586–595. [Google Scholar] [CrossRef]
- Miyashita, T.; Reed, J.C. Tumor Suppressor P53 Is a Direct Transcriptional Activator of the Human Bax Gene. Cell 1995, 80, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.-H.; Lee, J.-G.; Yang, Y.-I.; Kim, J.-H.; Ahn, J.-H.; Baek, N.-I.; Lee, K.-T.; Choi, J.-H. Eupatilin, a Dietary Flavonoid, Induces G2/M Cell Cycle Arrest in Human Endometrial Cancer Cells. Food Chem. Toxicol. 2011, 49, 1737–1744. [Google Scholar] [CrossRef]
- Wang, X.; Bai, H.; Zhang, X.; Liu, J.; Cao, P.; Liao, N.; Zhang, W.; Wang, Z.; Hai, C. Inhibitory Effect of Oleanolic Acid on Hepatocellular Carcinoma via ERK-P53-Mediated Cell Cycle Arrest and Mitochondrial-Dependent Apoptosis. Carcinogenesis 2013, 34, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Herrera, I.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Epicatechin Gallate Induces Cell Death via P53 Activation and Stimulation of P38 and JNK in Human Colon Cancer SW480 Cells. Nutr. Cancer 2013, 65, 718–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sui, Y.; Yao, H.; Li, S.; Jin, L.; Shi, P.; Li, Z.; Wang, G.; Lin, S.; Wu, Y.; Li, Y.; et al. Delicaflavone Induces Autophagic Cell Death in Lung Cancer via Akt/MTOR/P70S6K Signaling Pathway. J. Mol. Med. 2017, 95, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Wung, B.S.; Hsu, M.C.; Wu, C.C.; Hsieh, C.W. Resveratrol Suppresses IL-6-Induced ICAM-1 Gene Expression in Endothelial Cells: Effects on the Inhibition of STAT3 Phosphorylation. Life Sci. 2005, 78, 389–397. [Google Scholar] [CrossRef]
- Rahimi-Madiseh, M.; Malekpour-Tehrani, A.; Bahmani, M.; Rafieian-Kopaei, M. The Research and Development on the Antioxidants in Prevention of Diabetic Complications. Asian Pac. J. Trop. Med. 2016, 9, 825–831. [Google Scholar] [CrossRef] [Green Version]
- Akanji, M.A.; Fatinukun, H.D.; Rotimi, D.E.; Afolabi, B.L.; Adeyemi, O.S. The Two Sides of Dietary Antioxidants in Cancer Therapy; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Jaruga, P.; Birincioglu, M.; Rodriguez, H. Free Radical-Induced Damage to DNA: Mechanisms and Measurement. Free Radic. Biol. Med. 2002, 32, 1102–1115. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. CPB 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Tzvetkov, N.T.; El-Tawil, O.S.; Bungǎu, S.G.; Abdel-Daim, M.M.; Atanasov, A.G. Antioxidants: Scientific Literature Landscape Analysis. Oxid. Med. Cell. Longev. 2019, 2019, 8278454. [Google Scholar] [CrossRef] [PubMed]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative Stress and Dietary Phytochemicals: Role in Cancer Chemoprevention and Treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A Review of Anti-Cancer Properties and Therapeutic Activity in Head and Neck Squamous Cell Carcinoma. Mol. Cancer 2011, 10, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.-S.; Wu, J.-R.; Hu, C.-T. Signal Cross Talks for Sustained MAPK Activation and Cell Migration: The Potential Role of Reactive Oxygen Species. Cancer Metastasis Rev. 2008, 27, 303–314. [Google Scholar] [CrossRef]
- Dhawan, P.; Richmond, A. A Novel NF-Kappa B-Inducing Kinase-MAPK Signaling Pathway up-Regulates NF-Kappa B Activity in Melanoma Cells. J. Biol. Chem. 2002, 277, 7920–7928. [Google Scholar] [CrossRef] [Green Version]
- Das, L.; Vinayak, M. Long Term Effect of Curcumin in Regulation of Glycolytic Pathway and Angiogenesis via Modulation of Stress Activated Genes in Prevention of Cancer. PLoS ONE 2014, 9, e99583. [Google Scholar] [CrossRef] [Green Version]
- Garattini, E.; Bolis, M.; Garattini, S.K.; Fratelli, M.; Centritto, F.; Paroni, G.; Gianni’, M.; Zanetti, A.; Pagani, A.; Fisher, J.N.; et al. Retinoids and Breast Cancer: From Basic Studies to the Clinic and Back Again. Cancer Treat. Rev. 2014, 40, 739–749. [Google Scholar] [CrossRef]
- Doldo, E.; Costanza, G.; Agostinelli, S.; Tarquini, C.; Ferlosio, A.; Arcuri, G.; Passeri, D.; Scioli, M.G.; Orlandi, A. Vitamin A, Cancer Treatment and Prevention: The New Role of Cellular Retinol Binding Proteins. BioMed Res. Int. 2015, 2015, 624627. [Google Scholar] [CrossRef] [Green Version]
- Werner, B.; Gallagher, R.E.; Paietta, E.M.; Litzow, M.R.; Tallman, M.S.; Wiernik, P.H.; Slack, J.L.; Willman, C.L.; Sun, Z.; Traulsen, A.; et al. Dynamics of Leukemia Stem-like Cell Extinction in Acute Promyelocytic Leukemia. Cancer Res. 2014, 74, 5386–5396. [Google Scholar] [CrossRef] [Green Version]
- van Breemen, R.B.; Pajkovic, N. Multitargeted Therapy of Cancer by Lycopene. Cancer Lett. 2008, 269, 339–351. [Google Scholar] [CrossRef] [Green Version]
- Montagnani Marelli, M.; Marzagalli, M.; Fontana, F.; Raimondi, M.; Moretti, R.M.; Limonta, P. Anticancer Properties of Tocotrienols: A Review of Cellular Mechanisms and Molecular Targets. J. Cell. Physiol. 2019, 234, 1147–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constantinou, C.; Charalambous, C.; Kanakis, D. Vitamin E and Cancer: An Update on the Emerging Role of γ and δ Tocotrienols. Eur. J. Nutr. 2020, 59, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Stevens-Barrón, J.; Astiazarán-Garcia, H.; Robles-Zepeda, R.E. Efectividad y principales mecanismos anticancerígenos de tocotrienoles en líneas celulares malignas. Rev. Mex. Cienc. Farm. 2017, 13, 16–27. [Google Scholar]
- Shin-Kang, S.; Ramsauer, V.P.; Lightner, J.; Chakraborty, K.; Stone, W.; Campbell, S.; Reddy, S.A.G.; Krishnan, K. Tocotrienols Inhibit AKT and ERK Activation and Suppress Pancreatic Cancer Cell Proliferation by Suppressing the ErbB2 Pathway. Free Radic. Biol. Med. 2011, 51, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnekenburger, M.; Dicato, M.; Diederich, M. Plant-Derived Epigenetic Modulators for Cancer Treatment and Prevention. Biotechnol. Adv. 2014, 32, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Almoyad, M.; Huq, F. Polyphenols in Colorectal Cancer: Current State of Knowledge Including Clinical Trials and Molecular Mechanism of Action. BioMed Res. Int. 2018, 2018, 4154185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.-L.; Liu, Y.-Y.; Ma, Y.-G.; Xue, Y.-X.; Liu, D.-G.; Ren, Y.; Liu, X.-B.; Li, Y.; Li, Z. Curcumin Blocks Small Cell Lung Cancer Cells Migration, Invasion, Angiogenesis, Cell Cycle and Neoplasia through Janus Kinase-STAT3 Signalling Pathway. PLoS ONE 2012, 7, e37960. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, S.; Barbieri, A.; Palma, G.; Luciano, A.; Rea, D.; Arra, C. Curcumin Inhibits Tumor Growth and Angiogenesis in an Orthotopic Mouse Model of Human Pancreatic Cancer. BioMed Res. Int. 2013, 2013, 810423. [Google Scholar] [CrossRef]
- Murakami, C.; Hirakawa, Y.; Inui, H.; Nakano, Y.; Yoshida, H. Effect of Tea Catechins on Cellular Lipid Peroxidation and Cytotoxicity in HepG2 Cells. Biosci. Biotechnol. Biochem. 2002, 66, 1559–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.-H.; Li, Y.-Q.; Yang, X.-Y. Inhibition of Epigallocatechin Gallate on Orthotopic Colon Cancer by Upregulating the Nrf2-UGT1A Signal Pathway in Nude Mice. PHA 2007, 80, 269–278. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Mohammed, H.M.; Khadrawy, S.M.; Galaly, S.R. Hesperidin Protects against Chemically Induced Hepatocarcinogenesis via Modulation of Nrf2/ARE/HO-1, PPARγ and TGF-Β1/Smad3 Signaling, and Amelioration of Oxidative Stress and Inflammation. Chem. Biol. Interact. 2017, 277, 146–158. [Google Scholar] [CrossRef]
- Granado-Serrano, A.B.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Quercetin Modulates Nrf2 and Glutathione-Related Defenses in HepG2 Cells: Involvement of P38. Chem. Biol. Interact. 2012, 195, 154–164. [Google Scholar] [CrossRef] [Green Version]
- Ryu, M.J.; Chung, H.S. [10]-Gingerol Induces Mitochondrial Apoptosis through Activation of MAPK Pathway in HCT116 Human Colon Cancer Cells. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 92–101. [Google Scholar] [CrossRef]
- Lewinska, A.; Adamczyk-Grochala, J.; Kwasniewicz, E.; Deregowska, A.; Wnuk, M. Ursolic Acid-Mediated Changes in Glycolytic Pathway Promote Cytotoxic Autophagy and Apoptosis in Phenotypically Different Breast Cancer Cells. Apoptosis 2017, 22, 800–815. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Sridhar, J.; Foroozesh, M. Cytochrome P450 family 1 inhibitors and structure-activity relationships. Molecules 2013, 18, 14470–14495. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Rao, J.; Li, W.; Wang, K.; Qiu, F. Mechanism-based inactivation of cytochrome P450 enzymes by natural products based on metabolic activation. Drug Metab. Rev. 2020, 52, 501–530. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Gao, Q.; Zhang, T.; Rao, J.; Ding, L.; Qiu, F. Inhibition of CYP2C9 by natural products: Insight into the potential risk of herb-drug interactions. Drug Metab. Rev. 2020, 52, 235–257. [Google Scholar] [CrossRef]
- Ciolino, H.P.; Daschner, P.J.; Yeh, G.C. Resveratrol inhibits transcription of CYP1A1 in vitro by preventing activation of the aryl hydrocarbon receptor. Cancer Res. 1998, 58, 5707–5712. [Google Scholar] [PubMed]
- Casper, R.F.; Quesne, M.; Rogers, I.M.; Shirota, T.; Jolivet, A.; Milgrom, E.; Savouret, J.F. Resveratrol has antagonist activity on the aryl hydrocarbon receptor: Implications for prevention of dioxin toxicity. Mol. Pharmacol. 1999, 56, 784–790. [Google Scholar] [PubMed]
- Revel, A.; Raanani, H.; Younglai, E.; Xu, J.; Rogers, I.; Han, R.; Savouret, J.F.; Casper, R.F. Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects lung from DNA damage and apoptosis caused by benzo[a]pyrene. J. Appl. Toxicol. 2003, 23, 255–261. [Google Scholar] [CrossRef]



| Common Name | Botanical Name | Therapeutic Role | Type of Cancer | References |
|---|---|---|---|---|
| Indian Gooseberry | Phyllanthus emblica | Immunomodulatory, cytoprotective | Breast cancer | [33] |
| Garlic | Allium sativum | Anticancer, immuno-stimulant | Oesophageal, colorectal cancer | [34,35] |
| Turmeric | Curcuma longa | Anti-inflammatory, anticancer; chemo-resistance | Breast cancer | [36,37,38] |
| Common mayapple | Podophyllum peltatum | Anticancer | Testicular, lung cancer | [39] |
| Heartleaf moonseed plant | Tinospora cordifolia | Immunomodulatory, antioxidant, anticancer | Cervical cancer | [40] |
| King of bitters | Andrographis paniculata | Immune stimulator | Leukaemia, Colon, breast cancer | [41] |
| Atemoya | Annona atemoya | Anticancer | Lung, Colon, breast cancer | [42] |
| Stone breaker | Phyllanthus amarus | Cell cycle arrest, DNA repair, anti-angiogenic | Lung | [43] |
| Amruta | Mappia foetida | Antineoplastic | Leukaemia, lymphoma, cervical | [44] |
| Winter cherry | Withania somnifera | Anti-inflammatory, antitumor, antioxidant, immuno-modulatory | Leukaemia | [45] |
| Himalayan cedar | Cedrus deodara | Apoptosis induction | Leukaemia | [46] |
| Heart leaved moonseed | Tinospora cordifolia | Cytotoxic | Cervical | [47] |
| Soursop | Annona muricata | Cytotoxic | Breast | [48] |
| Chestnut rose | Rosa roxburghii | Immunomodulatory, antiaging | Oesophageal, gastric, pulmonary | [49] |
| Jewel Vine | Derris scandens | Radiosensitizer | Colon | [50] |
| Penawar Hitam | Goniothalamus macrophyllus | Apoptosis induction | Cervical | [51] |
| Dong quai | Angelica sinensis | Cytotoxic | Leukaemia | [52] |
| Cang Zhu | Atractylis lancea | Apoptotic, cell cycle arrest | Liver | [53] |
| Mongolian milkvetch | Astragalus membranaceus | Immunomodulatory | Myeloid Leukaemia | [54] |
| Tea plant | Camellia sinensis | Antioxidant, antitumor, antibacterial, antimutagenic | Breast, lung, colon, skin | [55] |
| Fire-flame bush | Woodfordia fruticosa | Cytotoxic | Lung, colon, Liver, Neuroblastoma | [56] |
| Red spiderling | Boerhaavia diffusa | Cytotoxic, anticarcinogenic | Cervical | [57] |
| Umbrella cheese tree | Glochidion zeylanicum | Cytotoxic | Prostate, liver, colon | [56] |
| Black nightshade | Solanum nigrum | Antimicrobial, antioxidant, cytotoxic, antiulcerogenic, hepatoprotective | Cervical | [58] |
| Chaga | Inonotus obliquus | Anticancer | Lung, breast, cervical, stomach | [59] |
| Chemopreventive Agents | Chemical Structure | Chemopreventive Mechanism | References |
|---|---|---|---|
| Curcumin |  | Inhibits lipid peroxidation, free radical generation, lipo- and cyclooxygenase, protein kinase C | [132] |
| Resveratrol |  | Alter signalling mechanism of cell division and proliferation, cell death, inflammation, angiogenesis, metastasis | [70] |
| Apigenin |  | Inhibit angiogenesis, malignant transformation, cell invasion, metastasis, induce apoptosis, regulate cell cycle | [133] |
| Epigallocatechin gallate |  | Regulate signalling pathways- PI3K/AKT, JAK/STAT, Notch, Wnt, AP-1, NFκB. tumor suppressor activities- p53; p21, p16 and Rb | [134] |
| Genistein |  | Inhibit cell growth, protein-tyrosine kinase, NF-κB, Akt signalling, PTK, regulate cell cycle regulation, induce apoptosis | [135] |
| Phytochemical | Action | Gene Regulation | References |
|---|---|---|---|
| Curcumin | Antiproliferation, antimigration, anti-invasion, cytotoxicity Reduces tumor invasiveness | ↓ ERK1/2 and NFkB STAT | [206,207] |
| Epigallocatechin-3-gallate | Antiproliferation, antioxidant defence capacity Reduces exogenous oxidative stress | ↓ NFkB ↑ GSH ↑ Nrf2, UGT1A, UGT1A8, and UGT1A10 | [208,209] |
| Resveratrol | Inhibits ROS-induced proliferation and Migration Protects against (4-OHE2)- induced migration and transformation | ↓ pERK, pAKT, and pNFkB ERK, NFkB and p38, MAPK/NFkB signaling | [210] |
| Hesperedin | Reducing oxidative stress | ↓ NFkB and COX-2 | [167] |
| Quercetin | Accelerates endogenous antioxidant enzymes, Apoptosis | GST and GPx | [211,212] |
| Gingerol | Apoptosis | ↑ MAPK | [213] |
| Ursolic acid | Antiproliferation, proapoptosis, proautophagy | ↓ Akt, ↓ MAPK | [171] |
| Apigenin, flavone, eupatilin | Antiproliferation, proapoptosis | ↓ Akt | [180] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
George, B.P.; Chandran, R.; Abrahamse, H. Role of Phytochemicals in Cancer Chemoprevention: Insights. Antioxidants 2021, 10, 1455. https://doi.org/10.3390/antiox10091455
George BP, Chandran R, Abrahamse H. Role of Phytochemicals in Cancer Chemoprevention: Insights. Antioxidants. 2021; 10(9):1455. https://doi.org/10.3390/antiox10091455
Chicago/Turabian StyleGeorge, Blassan P., Rahul Chandran, and Heidi Abrahamse. 2021. "Role of Phytochemicals in Cancer Chemoprevention: Insights" Antioxidants 10, no. 9: 1455. https://doi.org/10.3390/antiox10091455