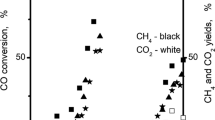
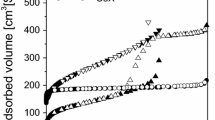
Abstract
Supported bimetallic catalysts of (Co + Pd)/SiO2 system were studied in carbon monoxide hydrogenation. Comparative analysis showed that depending on precursor treatment mode the catalysts ranged in different rows of activity in CO conversion and selectivity to methane. Samples of best performance were obtained after pretreatment in Ar flow. Highly selective catalysts were synthesized by reduction at 450 °C determining low metal dispersion, high extent of alloying, and agglomeration. A low H2,100C/COstrong adsorbed gas ratio was ascribed to a great amount of bimetallic particles and concerned with a diminished number of sites for multiply bonded CO species. Metal dispersion was low due to large Co particles, which enhanced CO dissociation and hydrogenation to CH4. In presence of bimetallic particles the reaction CO + 3H2 = CH4 + H2O was hampered. A decreased H2O formation influenced the WGS reaction. Catalyst samples activated at higher temperatures had better selectivity. During the process, formation of bidentate carbonate species was registered. It was supposed that palladium impeded creation of the latter species and following decomposition to CO2. Active catalyst samples were prepared by reduction at 300 °C leading to higher unreduced cobalt quota and metal dispersion, and decreased alloy particle formation. Higher H2,100C/COstrong ratio values were assigned to pure Co and Pd particle segregation, i.e. availability of sites for multiply bonded CO species favoring a higher activity in CO dissociation and further hydrogenation. A higher amount of CO species on these samples was conducive to CH4 formation, but also to CO2 production. The latter reaction was facilitated by unreduced cobalt.










Similar content being viewed by others
Data availability
All datasets for this study are included in the manuscript. They also are available in IC, BAS, Sofia, Bulgaria; IG, SAS, Kosice, Slovakia.
Code availability
Not applicable.
References
Owen EA, Jones DM (1954) Effect of grain size on the crystal structure of cobalt. Proc Phys Soc Sect B 67:456–466. https://doi.org/10.1088/0370-1301/67/6/302
Song D, Li J, Cai Q (2007) In situ diffuse reflectance FTIR study of CO adsorbed on a cobalt catalyst supported by silica with different pore sizes. J Phys Chem C 111:18970–18979. https://doi.org/10.1021/jp0751357
Floto ME, Ciufo RA, Han S, Mullins CB (2021) CO Dissociation on model Co/SiO2 catalysts—effect of adsorbed hydrogen. Surf Sci 705:121783. https://doi.org/10.1016/j.susc.2020.121783
Bidwell LR, Rizzo FE, Smith JV (1970) The thermodynamic properties of cobalt-palladium solid solutions. Acta Metall 18:1013–1019. https://doi.org/10.1016/0001-6160(70)90057-X
Zhang Q, Kang J, Wang Y (2010) Development of novel catalysts for Fischer-Tropsch synthesis: tunning the product selectivity. Chem Cat Chem 2:1030–1058. https://doi.org/10.1002/cctc.201000071
Murdoch A (2012) Structural and compositional analysis of Co–Pd model catalyst surfaces. PhD Thesis, University of St. Andrews
Lahtinen J, Vaari J, Kauraala K, Soares EA, Hove MAV (2000) LEED investigations on Co(0001): the (3x3)R30°—CO overlayer. Surf Sci 448:269–278. https://doi.org/10.1016/S0039-6028(99)01228-5
Toomes RL, King DA (1996) The adsorption of CO on Co{1010}. Surf Sci 349:1–18. https://doi.org/10.1016/0039-6028(95)01049-1
Gu J, Yeo Y, Sim WS, King DA (2000) Kinetic constraints in the phase transitions of chemisorbed carbon monoxide on Co{10-10} at high coverages. J Phys Chem B 104:4684–4689. https://doi.org/10.1021/jp994243t
Palazov A, Kadinov G, Bonev Ch, Shopov D (1987) Estimation of the number of CO molecules adsorbed in various modes on different crystal planes of alumina supported polycrystalline palladium. Surf Sci 188:505–518. https://doi.org/10.1016/S0039-6028(87)80202-9
Guo X, Yates JT (1989) Dependence of effective desorption kinetic parameters on surface coverage and adsorption temperature: CO on Pd(111). J Chem Phys 90:6761. https://doi.org/10.1063/1.456294
Giessel T, Schaff O, Hirschmugel CJ, Fernandez V, Schindler KM, Theobald A, Bao S, Lindsay R, Berndt W, Bradshaw AM, Baddleley D, Lee AF, Lambert RM, Woodruff DP (1998) A photoelectron diffraction study of ordered structures in the chemisorption system Pd{111}-CO. Surf Sci 406:90–102. https://doi.org/10.1016/S0039-6028(98)00098-3
Tüshaus T, Berndt W, Conrad H, Bradshaw AM, Persson B (1990) Understanding the structure of high coverage CO adlayers. Appl Phys A 51:91–98. https://doi.org/10.1007/BF00324270
Hansen KH, Worren T, Stempel S, Laegsgaard E, Baeumer M, Freund H-J, Besenbacher F, Stensgaard I (1999) Palladium nanocrystals on Al2O3: structure and adhesion energy. Phys Rev Lett 83:4120–4123. https://doi.org/10.1103/PhysRevLett.83.4120
Carlsson AF, Naschitzki M, Bäumer M, Freund H-J (2003) The structure and reactivity of Al2O3-supported cobalt−palladium particles: a CO-TPD, STM, and XPS study. J Phys Chem B 107:778–785. https://doi.org/10.1021/jp021966v
Carlsson AF, Bäumer M, Risse T, Freund H-J (2003) Surface structure of Co–Pd bimetallic particles supported on Al2O3 thin films studied using infrared reflection absorption spectroscopy of CO. J Chem Phys 119:10885–10894. https://doi.org/10.1063/1.1619943
Anastas PT, Kirchhoff MM, Williamson TC (2001) Catalysis as a foundational pillar of green chemistry. Appl Catal A 221:3–13. https://doi.org/10.1016/S0926-860X(01)00793-1
van Santen RA, Markvoort AJ, Filot IAW, Ghouri MM, Hensen EJM (2013) Mechanism and microkinetics of the Fischer-Tropsch reaction. Phys Chem Chem Phys 15:17038–17063. https://doi.org/10.1039/C3CP52506F
Zheng S, Liu Y, Li J, Shi B (2007) Deuterium tracer study of pressure effect on product distribution in the cobalt-catalyzed Fischer-Tropsch synthesis. Appl Catal A 330:63–68. https://doi.org/10.1016/j.apcata.2007.07.010
Oi LE, Choo M-Y, Lee HV, Ong HC, Hamid SBA, Juan JC (2016) Recent advances of titanium dioxide (TiO2) for green organic synthesis. RSC Adv 6:108741. https://doi.org/10.1039/C6RA22894A
Bunluesin T, Gorte RJ, Graham GW (1998) Studies of water-gas-shift reaction on ceria supported Pt, Pd and Rh: implications for oxygen-storage properties. Appl Catal B 15:107–114. https://doi.org/10.1016/S0926-3373(97)00040-4
Lin S, Ma J, Ye X, Xie D, Guo H (2013) CO hydrogenation on Pd(111): Competition between Fischer-Tropsch and oxygenate synthesis pathways. J Phys Chem C 117:14667–14676. https://doi.org/10.1021/jp404509v
Ma W, Jacobs G, Keogh RA, Bukur DB, Davis BH (2012) Fischer-Tropsch synthesis: Effect of Pd, Pt, Re, and Ru noble metal promoters on the activity and selectivity of a 25%Co/Al2O3 catalyst. Appl Catal A 437–438:1–9. https://doi.org/10.1016/j.apcata.2012.05.037
Anderson J (1978) Structure of metallic catalysts. Mir, Moscow (in Russian)
Ordomsky VV, Sushkevich VL, Ivanova II (2010) Study of acetaldehyde condensation chemistry over magnesia and zirconia supported on silica. J Mol Catal A 333:85–93. https://doi.org/10.1016/j.molcata.2010.10.001
Wang Y, Chen H, Zhao G, Liu M, Lang X, Zhu Z (2015) Influence of support properties on the activity of basic catalysts for aldol condensation of formaldehyde and methyl acetate in a continuous-flow reactor. J Flow Chem 5:87–94. https://doi.org/10.1556/JFC-D-14-00035
Chotiwan S, Tomiga H, Katagiri M, Yamamoto Y, Yamashita S, Katayama M, Inada Y (2016) Particle size effect of redox reactions for Co species supported on silica. J Solid State Chem 241:212–218. https://doi.org/10.1016/j.jssc.2016.06.020
Satterfield CN (1980) Heterogeneous catalysis in industrial practice. McGraw-Hill. Inc., New York
Wang Z-j, Skiles S, Yang F, Yan Z, Goodman DW (2012) Particle size effects in Fischer-Tropsch synthesis by cobalt. Catal Today 181:75–81. https://doi.org/10.1016/j.cattod.2011.06.021
Barbier A, Tuel A, Arcon I, Kodre A, Martin GA (2001) Characterization and catalytic behaviour of Co/SiO2 catalysts: influence of dispersion in the Fischer-Tropsch reaction. J Catal 200:106–116. https://doi.org/10.1006/jcat.2001.3204
Shirley D (1972) High-resolution X-ray photoemission spectrum of the valence bands of gold. Phys Rev B 5:4709–4714. https://doi.org/10.1103/PhysRevB.5.4709
Scofield JH (1976) Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J Electron Spectrosc Relat Phenom 8:129–137. https://doi.org/10.1016/0368-2048(76)80015-1
Reuel RC, Bartholomew CH (1984) The stoichiometries of H2 and CO adsorptions on cobalt: effects of support and preparation. J Catal 85:63–77. https://doi.org/10.1016/0021-9517(84)90110-6
Zowtiak JM, Bartholomew CH (1983) The kinetics of H2 adsorption on and desorption from cobalt and the effects of support thereon. J Catal 83:107–120. https://doi.org/10.1016/0021-9517(83)90034-9
Aben PC (1968) Palladium areas in supported catalysts: determination of palladium surface areas in supported catalysts by means of hydrogen chemisorption. J Catal 10:224–229. https://doi.org/10.1016/S0021-9517(68)80002-8
Todorova S, Zhelyazkov V, Kadinov G (1996) IR, TPR and chemisorption study of alumina-supported cobalt catalysts. Reac Kinet Catal Lett 57:105–110. https://doi.org/10.1007/BF02076127
Rashidi NA, Yusup S, Borhan A (2016) Isotherm and thermodynamic analysis of carbon dioxide on activated carbon. Proced Eng 148:630–637
Osmari TA, Gallon R, Schwaab M, Barbosa-Coutinho E, Severo JB Jr, Pinto JC (2013) Statistical analysis of linear and non-linear regression for the estimation of adsorption isotherm parameters. Adsorpt Sci Technol 31:433–458. https://doi.org/10.1260/0263-6174.31.5.433
Thomas JM, Tomas WJ (1969) Introduction to the principles of heterogeneous catalysis. Mir, Moscow (in Russian)
Adamson AW (1979) Physical chemistry of surfaces. Mir, Moscow (in Russian)
Barka N, Ouzaouit K, Abdennouri M, Makhfouk ME (2013) Dried prickly pear cactus (Opuntia ficus indica) cladodes as a low-cost and eco-friendly biosorbent for dyes removal from aqueous solutions. J Taiwan Inst Chem Eng 44:52–60. https://doi.org/10.1016/j.jtice.2012.09.007
Romero JRG, Moreno-Piraján JC, Gutierrez LG (2018) Kinetic and equilibrium study of the adsorption of CO2 in ultramicropores of resorcinol-formaldehyde aerogels obtained in acidic and basic medium. Carbon 4:52. https://doi.org/10.3390/c4040052
Madaeni SS, Salehi E (2009) Adsorption of cations on nanofiltration membrane: separation mechanism, isotherm confirmation and thermodynamic analysis. Chem Eng J 150:114–121. https://doi.org/10.1016/j.cej.2008.12.005
Shopska MG, Kadinov GB, Shtereva IZh (2011) Determination of cobalt oxide phases in Co- and Co–Pd supported catalyst pretreated in an inert atmosphere. Oxid Commun 34:85–91
Niemela MK, Backman L, Krause AOI, Vaara T (1997) The activity of the CoSiO2 catalyst in relation to pretreatment. Appl Catal 156:319–334. https://doi.org/10.1016/S0926-860X(97)00044-6
Heemeier M, Carlsson AF, Naschitzki M, Schmal M, Baeumer M, Freund H-J (2002) Preparation and characterization of a model bimetallic catalyst: Co–Pd nanoparticles supported on Al2O3. Angew Chem Int Ed 41:4073–4076. https://doi.org/10.1002/1521-3773(20021104)41:21%3c4073::AID-ANIE4073%3e3.0.CO;2-M
Paredes-Nunez A, Jbir I, Bianchi D, Meunier FC (2015) Spectrum baseline artefacts and correction of gas-phase species signal during diffuse reflectance FT-IR analyses of catalysts at variable temperatures. Appl Catal A 495:17–22. https://doi.org/10.1016/j.apcata.2015.01.042
Tuxen A, Carenco S, Chintapalli M, Chuang C-H, Escudero C, Pach E, Jiang P, Borondics F, Beberwyck B, Alivisatos AP, Thornton G, Pong W-F, Guo J, Perez R, Besenbacher F, Salmeron M (2013) Size-dependent dissociation of carbon monoxide on cobalt nanoparticles. J Am Chem Soc 135:2273–2278. https://doi.org/10.1021/ja3105889
Sun X, Sartipi S, Kapteijn F, Gascon J (2016) Effect of pretreatment atmosphere on the activity and selectivity of Co/mesoHZSM-5 for Fischer-Tropsch synthesis. New J Chem 40:4167–4177. https://doi.org/10.1039/C5NJ02462E
Yu L-h, Zhang S-m, Guo X, Wang D, Wang S-r, Wu S-h (2007) Influence of the addition of Pd and Cu to cobalt catalysts prepared by SMAI for F-T synthesis. Cent Eur J Chem 5:144–155. https://doi.org/10.2478/s11532-006-0062-9
Masuda M (1976) Application of the theory of stoichiometric number determination of the rate-determining step: the mechanism of water-gas shift reaction catalyzed by platinum. J Res Inst Catal Hokkaido Univ 24:83
Raganati F, Alfe M, Gargiulo V, Chirone R, Ammendola P (2018) Isotherms and thermodynamics of CO2 adsorption on a novel carbon-magnetite composite sorbent. Chem Eng Res Des 134:540–552. https://doi.org/10.1016/j.cherd.2018.04.037
Ladshow A, Yiacoumi S, Tsouris C, DePaoli D (2015) Generalized gas–solid adsorption modeling: single-component equilibria. Fluid Phase Equilib 388:169–181. https://doi.org/10.1016/j.fluid.2015.01.003
Swenson H, Stadie NP (2019) Langmuir’s theory of adsorption: a centennial review. Langmuir 35:5409–5426. https://doi.org/10.1021/acs.langmuir.9b00154
Guskos N, Typek J, Maryniak M, Żolnierkiewicz G, Podsiadly M, Arabczyk W, Lendzion-Bielun Z, Narkiewicz U (2006) Effect of calcination and structural additives on the EPR spectra of nanocrystalline cobalt oxides. Mater Sci-Pol 24:4
Stefanov P, Todorova S, Naydenov A, Tzaneva B, Kolev H, Atanasova G, Stoyanova D, Karakirova Y, Aleksieva K (2015) On the development of active and stable Pd-Co/γAl2O3 catalyst for complete oxidation of methane. Chem Eng J 266:329–338. https://doi.org/10.1016/j.cej.2014.12.099
Popova NM, Babenkova LV, Savel’eva GA (1979) Adsorption and interaction of simple gases with VIIIth group metals. Nauka, Kaz. SSR, Alma-Ata (in Russian)
Potoczna-Petru D, Jablonski JM, Okal J, Krajczyk L (1998) Influence of oxidation-reduction treatment on microstructure of Co/SiO2 catalysts. Appl Catal A 175:113–120. https://doi.org/10.1016/S0926-860X(98)00214-2
Shopska M, Kadinov G, Shtereva I (2014) Determination of metal dispersion in cobalt-palladium catalysts. Rev Roum Chim 59:219–225
Moulder JF, Stickle WF, Sobol PE, Bomben KD (1992) Handbook of X-ray photoelectron spectroscopy. Perkin-Elmer Corp, Minnesota, USA
Sun S, Fujimoto K, Yoneyama Y, Tsubaki N (2002) Fischer-Tropsch synthesis using Co/SiO2 catalysts prepared from mixed precursors and addition effect of noble metals. Fuel 81:1583–1591. https://doi.org/10.1016/S0016-2361(02)00090-X
Chen YW, Wang HT, Goodwin JG (1983) Effect of preparation methods on the catalytic properties of zeolite-supported ruthenium in the Fischer-Tropsch synthesis. J Catal 83:415–427. https://doi.org/10.1016/0021-9517(83)90066-0
Gnanamani MK, Jacobs G, Pendyala VRR, Graham UM, Hopps SD, Thomas GA, Shafer WD, Sparks DE, Xiao Q, Hu Y, Davis BH (2016) Fischer-Tropsch synthesis: anchoring of cobalt particles in phosphorus modified cobalt/silica catalysts. Appl Catal A 523:146–158. https://doi.org/10.1016/j.apcata.2016.05.024
Zhou W, Chen J-G, Fang K-G, Sun Y-H (2006) The deactivation of Co/SiO2 catalyst for Fischer-Tropsch synthesis at different ratios of H2 to CO. Fuel Proc Technol 87:609–616. https://doi.org/10.1016/j.fuproc.2006.01.008
Singh JA, Yang N, Liu X, Tsai C, Stone KH, Johnson B, Koh AL, Bent SF (2018) Understanding the active sites of CO hydrogenation on Pt-Co catalysts prepared using atomic layer deposition. J Phys Chem C 122:2184–2194. https://doi.org/10.1021/acs.jpcc.7b10541
Kumar N, Smith ML, Spivey J (2012) Characterization and testing of silica-supported cobalt–palladium catalysts for conversion of syngas to oxygenates. J Catal 289:218–226. https://doi.org/10.1016/j.jcat.2012.02.011
Kadinov G, Bonev Ch, Todorova S, Palazov A (1998) IR spectroscopy study of CO adsorption and of the interaction between CO and hydrogen on alumina supported cobalt. J Chem Soc Faraday Trans 94:3027–3031. https://doi.org/10.1039/A804315I
Lapidus A, Krylova A, Kazanski V, Borovkov V, Zaitsev A, Rathousky J, Zukal A, Jančalkova M (1991) Hydrocarbon synthesis from carbon monoxide and hydrogen on impregnated cobalt catalysts Part I. Physico-chemical properties of 10% cobalt/alumina and 10% cobalt/silica. Appl Catal 73:65–81. https://doi.org/10.1016/0166-9834(91)85113-A
Hadjiivanov K, Vaisilov G (2002) Characterization of oxide surfaces and zeolites by carbon monoxide as an IR probe molecule. Adv Catal 47:307–511. https://doi.org/10.1016/S0360-0564(02)47008-3
Tsubaki N, Sun S, Fujimoto K (2001) Different functions of the noble metals added to cobalt catalysts for Fischer-Tropsch synthesis. J Catal 199:236–246. https://doi.org/10.1006/jcat.2001.3163
Little LH (1966) Infrared spectra of adsorbed species. Academic Press Inc., London
Blyholder G (1964) Molecular orbital view of chemisorbed carbon monoxide. J Phys Chem 68:2772–2777. https://doi.org/10.1021/j100792a006
Bian G, Nanba T, Koizumi N, Yamada M (2002) Changes in microstructure of a reduced cobalt catalyst during performing FT synthesis from syngas determined by in situ high-pressure syngas adsorption. J Mol Catal A 178:219–228. https://doi.org/10.1016/S1381-1169(01)00340-5
Mothebe B, Duvenhage DJ, Sokolovskii VD, Coville NJ (1997) DRIFTS studies on Co/TiO2 Fischer-Tropsch catalysts. In: de Pontes M, Espinoza RL, Nicolaides CP, Scholtz JH, Scurrell MS (eds) Studies in surface science and catalysis. (Natural gas conversion IV), vol 107. Elsevier, Amsterdam, pp 187–192. https://doi.org/10.1016/S0167-2991(97)80333-3
Szanyi J, Kwak JH (2014) Dissecting the steps of CO2 reduction: 1. The interaction of CO and CO2 with γ-Al2O3: an in situ FTIR study. Phys Chem Chem Phys 16:15117–15125. https://doi.org/10.1039/C4CP00616J
Sethuraman R, Bakhshi NN, Katikaneni SP, Idem RO (2001) Production of C4 hydrocarbons from Fischer-Tropsch synthesis in a follow bed reactor consisting of Co–Ni–ZrO2 and sulfated-ZrO2 catalyst beds. Fuel Proc Technol 73:197–222. https://doi.org/10.1016/S0378-3820(01)00199-0
Noronha FB, Schmal M, Moraweck B, Delichere P, Brun M, Villain F, Frety R (2000) Characterization of niobia-supported palladium-cobalt catalysts. J Phys Chem B 104:5478–5485. https://doi.org/10.1021/jp992777o
Mallat T, Szabo S, Petro J, Mendioroz S, Folgado MA (1989) Real and apparent dispersion of carbon supported palladium-cobalt catalysts. Appl Catal 53:29–40. https://doi.org/10.1016/S0166-9834(00)80007-X
Savitskii EM, Polyakova VP, Gorina NB, Roshan NR (1975) Metallurgy of platinum metals. Metallurgy, Moscow (in Russian)
Juszczyk W, Karpinski Z, Lomot D, Pielaszek J, Paal Z, Stakheev AYu (1993) The structure and activity of silica-supported palladium-cobalt alloys I. Alloy homogeneity, surface composition, and activity for neopentane conversion. J Catal 142:617–629. https://doi.org/10.1006/jcat.1993.1235
Kaszkur ZA, Mierzwa B (1998) Segregation in model palladium-cobalt clusters. Philos Mag A 77:781–800. https://doi.org/10.1080/01418619808224084
Mierzwa B, Kaszkur Z, Moraweck B, Pielaszek J (1999) In situ EXAFS study of the alloy catalyst Pd-Co (50%/50%)/SiO2. J Alloys Compd 286:93–97. https://doi.org/10.1016/S0925-8388(98)00986-4
Matolinova I, Fabik S, Masek K, Sedlacek L, Skala T, Veltruska K, Matolin V (2003) Influence of Pd–Co bimetallic interaction on CO adsorption properties of PdxCo1−x alloys: XPS, TPD and static SIMS studies. Vacuum 71:41–45. https://doi.org/10.1016/S0042-207X(02)00711-X
Viswanathan B, Gopalakrishnan R, Vetrivel R (1982) Temperature programmed desorption (TPD) of carbon monoxide from cobalt surfaces. React Kinet Catal Lett 18:209–212. https://doi.org/10.1007/BF02065165
Dropsch H, Baerns M (1997) CO adsorption on supported Pd catalysts studied by adsorption microcalorimetry and temperature programmed desorption. Appl Catal A 158:163–183. https://doi.org/10.1016/S0926-860X(96)00418-8
Suzuki T, Tajima S (2010) Enhancement of FT reaction on silica supported cobalt catalyst. Trans Mater Res Soc Jpn 35:635–638. https://doi.org/10.14723/tmrsj.35.635
Rabo A, Risch A, Poutsma M (1978) Reactions of carbon monoxide and hydrogen on Co, Ni, Ru, and Pd metals. J Catal 53:295–311. https://doi.org/10.1016/0021-9517(78)90102-1
Sarkany A, Zsoldos Z, Stefler G, Hightower JW, Guczi L (1995) Promoter effect of Pd in hydrogenation of 1,3-butadiene over Co–Pd catalysts. J Catal 157:179–189. https://doi.org/10.1006/jcat.1995.1278
Chen T-Y, Su J, Zhang Z, Cao C, Wang X, Si R, Liu X, Shi B, Xu J, Han Y-F (2018) Structure evolution of Co-CoOx interface for higher alcohol synthesis from syngas over Co/CeO2 catalysts. ACS Catal 8:8606–8617. https://doi.org/10.1021/acscatal.8b00453
Rygh LES, Ellestad OH, Klæboe P, Nielsen CJ (2000) Infrared study of CO adsorbed on Co/γ-Al2O3 based Fischer-Tropsch catalysts; semi-empirical calculations as a tool for vibrational assignments. Phys Chem Chem Phys 2:1835–1846. https://doi.org/10.1039/B000188K
Davis BH, Iglesia E (2002) Technology development for iron and cobalt Fischer-Tropsch catalysts, final technical report DE-FC26-98FT40308. University of California at Berkeley & University of Kentucky Research Foundation
Fan L, Zhang L, Shen Y, Liu D, Wahab N, Hasan MM (2016) Liquid-phase hydrogenation of phenol to cyclohexanone over supported palladium catalysts. Bull Chem React Eng Catal 11:354–362. https://doi.org/10.9767/bcrec.11.3.575.354-362
Iloy RA, Jalama K (2019) Effect of operating temperature, pressure and potassium loading on the performance of silica-supported cobalt catalyst in CO2 hydrogenation to hydrocarbon fuel. Catalysts 9:807. https://doi.org/10.3390/catal9100807
Qin H, Qian X, Meng T, Lin Y, Ma Z (2015) Pt/MOx/SiO2, Pt/MOx/TiO2, and Pt/MOx/Al2O3 catalysts for CO oxidation. Catalysis 5:606–633. https://doi.org/10.3390/catal5020606
Grenoble DC, Estadt MM, Ollis DF (1981) The chemistry and catalysis of the water gas shift reaction: 1. The kinetics over supported metal catalysts. J Catal 67:90–102. https://doi.org/10.1016/0021-9517(81)90263-3
Acknowledgements
Financial support by Bulgarian National Science Fund through contract KP-06-H29-9/14.12.2018 is greatly acknowledged. Research equipment of Distributed research infrastructure INFRAMAT (part of Bulgarian National Roadmap for Research Infrastructures) supported by Bulgarian Ministry of Education and Science was used in this investigation. The authors acknowledge English language text editing by Assoc. Prof. C. Bonev.
Funding
The research leading to these results received funding from Bulgarian National Science Fund under Grant Agreement No. KP-06-H29-9/2018.
Author information
Authors and Affiliations
Contributions
Conceptualization, writing—original draft preparation, visualization, project administration, funding acquisition [MS]; methodology [MS, ST, GK]; validation [MS, ST, HK, MF, KA, KT]; investigation [MS, HK, MF, KA, KT, ST, GK]; resources [MS, ST]; data curation [MS, ST, HK, MF, KA, KT]; writing—review and editing [GK]; supervision [GK].
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
Ethics approval was not required for this research.
Consent for publication
All authors have read and agreed to the published version of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shopska, M., Kolev, H., Aleksieva, K. et al. Study of sites and species during CO hydrogenation over silica-supported Co–Pd catalysts. Relation to performance in the process. Reac Kinet Mech Cat 134, 303–330 (2021). https://doi.org/10.1007/s11144-021-02067-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02067-9