Abstract
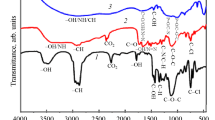
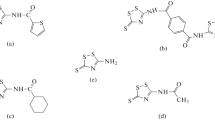
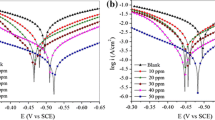
The achievement of high corrosion inhibition performances by manipulating the molecular structure of organic substances has gained much attention in recent years. In this paper, POCl3 was synthesized and Cl atoms in phosphoryl chloride (POCl3) were replaced by aniline or phenyl hydrazine and their derivatives; the effect of the substitution on the corrosion inhibition performance for mild steel immersed in 1 M HCl solution was investigated. Fourier transform infrared spectroscopy (FT-IR), nuclear magnetic resonance (NMR) and ultraviolet-visible (UV–Vis) spectroscopy were used to determine the molecular structure of the synthesized organic substances. Electrochemical impedance spectroscopy (EIS) and polarization measurements were employed to investigate the corrosion inhibition performance of the organic molecules for the immersed mild steel samples in the acid solution. The obtained results indicated that the inhibition efficiency of the synthesized inhibitors was increased by increasing the molecular size of the organic substances as well as increasing the number of nitrogen and oxygen heteroatoms in the structure of the organic molecules. The maximum amount of inhibition efficiency of the synthesized organic inhibitors in this paper was obtained about 93%.













Similar content being viewed by others
REFERENCES
Merello, R., Botana, F.J., Botella, J., Matres, M.V., and Marcos, M., Corros. Sci., 2003, vol. 45, pp. 909–921.
Cunat, P.-J., Alloying Elements in Stainless Steel, Euro Inox, 2004, pp. 1–24.
Javidparvar, A.A., Naderi, R., Ramezanzadeh, B., and Bahlakeh, G., J. Ind. Eng. Chem., 2019, vol. 72, pp. 196–213.
Saji, V.S., Recent Pat. Corros. Sci., 2010, vol. 2, pp. 6–12.
Javidparvar, A.A., Ramezanzadeh, B., and Ghasemi, E., Inst. Color Sci. Technol., 2015, vol. 10, p. 12.
Javidparvar, A.A., Ramezanzadeh, B., and Ghasemi, E., J. Taiwan Inst. Chem. Eng., 2015, vol. 61, pp. 356–366.
Dayal, R.K., in Corrosion of Austenitic Stainless Steels, Cambridge: Woodhead Publ., 2002.
Hughes, A.E., Ho, D., Forsyth, M., and Hinton, B.R.W., Corros. Rev., 2007, vol. 25, pp. 591–605.
Francis, R. and Powell, C., Corrosion and Anti-Corrosives, European Federation of Corrosion, 2012.
Atabaki, F., Jahangiri, Sh., and Pahnavar, Z., Prot. Met. Phys. Chem. Surf., 2019, vol. 55, pp. 1161–1172.
Atabaki, F., Bastam, N.N., Hafizi-Atabak, H., Radvar, M., and Jahangiri, Sh., Iran. J. Chem. Chem. Eng., 2020, vol. 39, no. 4, p. 113.
Fink, J., Petroleum Engineer’s Guide to Oil Field Chemicals and Fluids, Gulf Professional Publ., 2015, pp. 215–254.
Dariva, C.G. and Galio, A.F., Corrosion Inhibitors - Principles, Mechanisms and Applications, IntechOpen, 2014, pp. 365–379.
Somers, A.E., Hinton, B.R.W., De Bruin-Dickason, C., Deacon, G.B., Junk, P.C., and Forsyth, M., Corros. Sci., 2018, vol. 139, pp. 430–437.
Palou, R.M., Olivares-Xomelt, O., and Likhanova, N.V., Environmentally friendly corrosion inhibitors, in Developments in Corrosion Protection, Aliofkhazraei, M., Ed., IntechOpen, 2014, pp. 431–465.
Arthur, D.E., Jonathan, A., Ameh, P.O., and Anya, C., Int. J. Ind. Chem., 2013, vol. 4, p. 2.
Wang, L., Qu, M.-Q., Yang, Y.-J., Peng, L., and Ma, S.-M., Int. J. Electrochem. Sci., 2016, vol. 11, pp. 9307–9325.
Org, W.E., Zhang, C., and Zhao, J., Int. J. Electrochem. Sci., 2017, vol. 12, pp. 9161–9179.
Brycki, B.E., Kowalczyk, H.I., Szulc, A., Kaczerewska, O., and Pakiet, M., Organic corrosion inhibitors, in Corrosion Inhibitors, Principles and Recent Applications, IntechOpen, 2018, pp. 3–30.
Rani, B.E.A. and Basu, B.B.J., Int. J. Corros., 2012, vol. 2012, pp. 1–15.
Ahmed, M.H.O., Al-Amiery, A.A., Al-Majedy, Y.K., Kadhum, A.A.H., Mohamad, A.B., and Gaaz, T.S., Results Phys., 2018, vol. 8, pp. 728–733.
Chai, C., Xu, Y., Li, D., Zhao, X., Xu, Y., Zhang, L., et al., Prog. Org. Coat., 2019, vol. 129, pp. 159–170.
Jiang, L., Qiang, Y., Lei, Z., Wang, J., Qin, Z., and Xian, B.G., J. Mol. Liq., 2018, vol. 255, pp. 53–63.
Öztürk, S., Yıldırım, A., Çetin, M., and Tavaslı, M., J. Surfactants Deterg., 2014, vol. 17, pp. 471–481.
Atabaki, F. and Jahangiri, Sh., J. Appl. Chem., 2017, vol. 11, pp. 67–74.
Moller, G., US Patent 3406013, 1968.
Draeger, A.G. et al., US Patent 3052520, 1962.
Gholivand, K., Pooyan, M., Mohamadpanah, F., Pirastefar, F., Junk, P.C., Wang, J., Ebrahimi Valmoozi, A.A., and Mani-Varnosfaderani, A., Bioorg. Chem., 2019, vol. 86, pp. 482–493.
Tarahhomi, A. and Van Der Lee, A., J. Coord. Chem., 2018, vol. 8972, pp. 1–26.
Son, Y.R. and Park, S.J., Sci. Rep., 2018, vol. 8, pp. 2–11.
Zou, S., Li, R., Kobayashi, H., Liu, J., and Fan, J., Chem. Commun., 2013, vol. 49, p. 1906.
Ozaki, Y., Morisawa, Y., Ikehata, A., and Higashi, N., Appl. Spectrosc. Rev., 2012, vol. 66, pp. 1–25.
Khaled, K.F., Electrochim. Acta, 2010, vol. 55, pp. 6523–6532.
Khaled, K.F. and Amin, M.A., J. Appl. Electrochem., 2009, vol. 39, pp. 2553–2568.
Javidparvar, A.A., Naderi, R., and Ramezanzadeh, B., J. Mol. Liq., 2019, vol. 284, pp. 415–430.
Asadi, N., Naderi, R., and Mahdavian, M., Prog. Org. Coat., 2019, vol. 132, pp. 29–40.
Javidparvar, A.A., Ramezanzadeh, B., and Ghasemi, E., Corrosion, 2016, vol. 72, pp. 1–49.
Javidparvar, A.A., Naderi, R, and Ramezanzadeh, B., Composites, Part B, 2019, vol. 172, pp. 363–375.
Chaudhry, A.U., Mittal, V., and Mishra, B., Mater. Chem. Phys., 2015, vol. 163, pp. 130–137.
Hirschorn, B., Orazem, M.E., Tribollet, B., Vivier, V., Frateur, I., and Musiani, M., Electrochim. Acta, 2010, vol. 55, pp. 6218–6227.
Labjar, N., Lebrini, M., Bentiss, F., Chihib, N.E., El Hajjaji, S., and Jama, C., Mater. Chem. Phys., 2010, vol. 119, pp. 330–336.
Li, X., Wang, Z., Li, Q., Ma, J., and Zhu, M., Chem. Eng. J., 2015, vol. 273, pp. 630–637.
Touir, R., Belakhmima, R.A., Ebn Touhami, M., Lakhrissi, L., El Fayed, M., Lakhrissi, B., et al., J. Mater. Environ. Sci., 2013, vol. 4, pp. 921–930.
Lim, S.H., Zeng, K.Y., and He, C.B., Mater. Sci. Eng., A, 2010, vol. 527, pp. 5670–5676.
Mahdavian, M. and Ashhari, S., Electrochim. Acta, 2010, vol. 55, pp. 1720–1724.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fariborz Atabaki, Shahrzad Jahangiri Thermodynamic and Electrochemical Studies of Aniline and Phenylhydrazine and Their Derivatives Substituted POCl3-Based Compounds as Corrosion Inhibitor for Mild Steel in Hydrochloric Acid Solution. Prot Met Phys Chem Surf 57, 820–833 (2021). https://doi.org/10.1134/S2070205121040055
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205121040055