Synergy of Hydeal-D® and Hyaluronic Acid for Protecting and Restoring Urothelium: In Vitro Characterization
Abstract
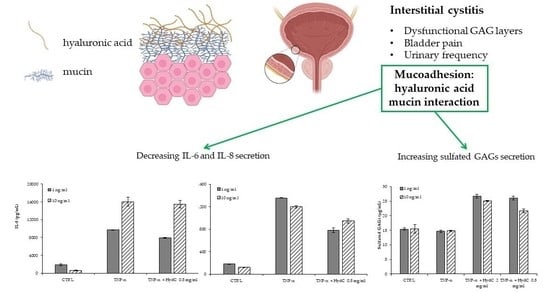
:1. Introduction
2. Experimental Part
2.1. Materials
2.2. Methods
2.2.1. Viscosity and Rheological Synergism
2.2.2. Washability Measurement
Active Substances Quantification
2.2.3. In Vitro Cytotoxicity Assay
2.2.4. In Vitro Efficacy Assay in an Inflammatory Model
2.2.5. Sulfated Glycosaminoglycans Assay
2.2.6. Statistical Analysis
3. Results and Discussion
3.1. Rheological Analysis and Rheological Synergism
3.2. Washability Properties
3.3. Cytotoxicity Assay
3.4. ELISA Assay Evaluating Cytokines Levels in T24 Supernatants
3.5. Secretion of sGAGs by Urothelial Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, E.Q.; Birnbaum, H.; Mareva, M.; Parece, A.; Huang, Z.; Mallett, D.; Taitel, H. Interstitial Cystitis: Cost, treatment and co-morbidities in an employed population. Pharmacoeconomics 2006, 24, 55–65. [Google Scholar] [CrossRef]
- Nickel, J.C.; Tripp, D.A.; Pontari, M.; Moldwin, R.; Mayer, R.; Carr, L.K.; Doggweiler, R.; Yang, C.C.; Mishra, N.; Nordling, J. Psychosocial phenotyping in women with interstitial cystitis/painful bladder syndrome: A case control study. J. Urol. 2010, 183, 167–172. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Weng, S.F.; Hsu, Y.W.; Huang, C.L.; Wu, M.P. Increased risks of healthcare-seeking behaviors of anxiety, depression and insomnia among patients with bladder pain syndrome/interstitial cystitis: A nationwide population-based study. Int. Urol. Nephrol. 2015, 47, 275–281. [Google Scholar] [CrossRef]
- Parsons, C.L. Bladder surface glycosaminoglycan: Efficient mechanism of environmental adaptation. Urology 1986, 27, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Janssen, D.A.; van Wijk, X.M.; Jansen, K.C.; van Kuppevelt, T.H.; Heesakkers, J.P.; Schalken, J.A. The distribution and function of chondroitin sulfate and other sulfated glycosaminoglycans in the human bladder and their contribution to the protective bladder barrier. J. Urol. 2013, 189, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Klingler, C.H. Glycosaminoglycans: How much do we know about their role in the bladder? Urologia 2016, 25, 83. [Google Scholar] [CrossRef]
- Patnaik, S.S.; Laganà, A.S.; Vitale, S.G.; Butticè, S.; Noventa, M.; Gizzo, S.; Valenti, G.; Rapisarda, A.M.C.; La Rosa, V.L.; Magno, C.; et al. Etiology, pathophysiology and biomarkers of interstitial cystitis/painful bladder syndrome. Arch. Gynecol. Obstet. 2017, 295, 1341–1359. [Google Scholar] [CrossRef]
- Cervigni, M.; Natale, F.; Nasta, L.; Padoa, A.; Voi, R.L.; Porru, D. A combined intravesical therapy with hyaluronic acid and chondroitin for refractory painful bladder syndrome/interstitial cystitis. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2008, 19, 943–947. [Google Scholar] [CrossRef]
- Hurst, R.E.; Rhodes, S.W.; Adamson, P.B.; Parsons, C.L.; Roy, J.B. Functional and Structural Characteristics of the Glycosaminoglycans of the Bladder Luminal Surface. J. Urol. 1987, 138, 433–437. [Google Scholar] [CrossRef]
- Teo, K.S.H.; Biers, S. Intravesical Glycosaminoglycan Analogue Instillations for Recurrent Cystitis. Available online: https://www.urologynews.uk.com/features/features/post/intravesical-glycosaminoglycan-analogue-instillations-for-recurrent-cystitis (accessed on 7 September 2021).
- Cervigni, M. Interstitial cystitis/bladder pain syndrome and glycosaminoglycans replacement therapy. Transl. Androl. Urol. 2015, 4, 638–642. [Google Scholar] [CrossRef]
- Lazzeri, M.; Hurle, R.; Casale, P.; Buffi, N.; Lughezzani, G.; Fiorini, G.; Peschechera, R.; Pasini, L.; Zandegiacomo, S.; Benetti, A.; et al. Managing chronic bladder diseases with the administration of exogenous glycosaminoglycans: An update on the evidence. Ther. Adv. Urol. 2016, 8, 91–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madersbacher, H.; van Ophoven, A.; van Kerrebroeck, P.E. GAG layer replenishment therapy for chronic forms of cystitis with intravesical glycosaminoglycans--a review. NeuroUrol. Urodyn. 2013, 32, 9–18. [Google Scholar] [CrossRef]
- Iavazzo, C.; Athanasiou, S.; Pitsouni, E.; Falagas, M.E. Hyaluronic acid: An effective alternative treatment of interstitial cystitis, recurrent urinary tract infections, and hemorrhagic cystitis? Eur. Urol. 2007, 51, 1534–1540. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Takahashi, T.; Ide, H.; Fukushima, T.; Tabata, M.; Sekine, F.; Kobayashi, K.; Negishi, M.; Niwa, Y. Antioxidant activity of synovial fluid, hyaluronic acid, and two subcomponents of hyaluronic acid. Synovial fluid scavenging effect is enhanced in rheumatoid arthritis patients. Arthritis Rheum. 1988, 31, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.L.; Toole, B.P. Hyaluronate inhibition of cell proliferation. Arthritis Rheum. 1987, 30, 769–778. [Google Scholar] [CrossRef]
- Brown, J.A. 2004. The role of hyaluronic acid in wound healing’s proliferative phase. J. Wound Care 2004, 13, 48–51. [Google Scholar] [CrossRef]
- Rooney, P.; Srivastava, A.; Watson, L.; Quinlan, L.R.; Pandit, A. Hyaluronic acid decreases IL-6 and IL-8 secretion and permeability in an inflammatory model of interstitial cystitis. Acta Biomater. 2015, 19, 66–75. [Google Scholar] [CrossRef]
- Boucher, W.S.; Letourneau, R.; Huang, M.; Kempuraj, D.; Green, M.; Sant, G.R.; Theoharides, T.C. Intravesical sodium hyaluronate inhibits the rat urinary mast cell mediator increase triggered by acute immobilization stress. J. Urol. 2002, 167, 380–384. [Google Scholar] [CrossRef]
- Liu, S.B.; Liu, S.L.; Gan, X.L.; Zhou, Q.; Hu, L.N. The effects of hyaluronic acid vaginal gel on the vaginal epithelium of ovariectomized rats. Gynecol. Endocrinol. 2015, 31, 208–213. [Google Scholar] [CrossRef]
- Della Valle, F.; Romeo, A. Esters of Hyaluronic Acid. U.S. Patent 4,851,521, 25 July 1989. [Google Scholar]
- Pizzocaro, C.; Zanellato, A.M.; Pavan, M. Pharmaceutical Composition for Use in the Treatment of Cystitis of Various Etiologies. WO2021079303, 29 April 2021. [Google Scholar]
- Rossi, S.; Vigani, B.; Bonferoni, M.C.; Sandri, G.; Caramella, C.; Ferrari, F. Rheological analysis and mucoadhesion: A 30 year-old and still active combination. J. Pharm. Biomed. Anal. 2018, 156, 232–238. [Google Scholar] [CrossRef]
- Perotto, G.; Sandri, G.; Pignatelli, C.; Milanesi, G.; Athanassiou, A. Water-based synthesis of keratin micro- and nanoparticles with tunable mucoadhesive properties for drug delivery. J. Mater. Chem. B 2019, 7, 4385–4392. [Google Scholar] [CrossRef]
- Rossi, S.; Bonferoni, M.C.; D’Autilia, F.; Sandri, G.; Ferrari, F.; Caramella, C.; Mortara, E.; Giannini, V.; Gasparri, F. Associations of natural polymers to modulate mucoadhesion of vaginal rinse-off and leave-on formulations. J. Drug Deliv. Sci. Technol. 2014, 24, 496–502. [Google Scholar] [CrossRef]
- Soato, M.; Galesso, D.; Beninatto, R.; Bettella, F.; Guarise, C.; Pavan, M. A versatile and robust analytical method for hyaluronan quantification in crosslinked products and complex matrices. Carbohydr. Res. 2021, 503, 108314. [Google Scholar] [CrossRef] [PubMed]
- Welsh, E.J.; Rees, D.A.; Morris, E.R.; Madden, J.K. Competitive Inhibition Evidence for Specific Intermolecular Interactions in Hyaluronate Solutions. J. Mol. Biol. 1980, 138, 375–382. [Google Scholar] [CrossRef]
- Hansen, I.M.; Ebbesen, M.F.; Kaspersen, L.; Thomsen, T.; Bienk, K.; Cai, Y.; Malle, B.M.; Howard, K. A Hyaluronic Acid Molecular Weight-Dependent Modulation of Mucin Nanostructure for Potential Mucosal Therapeutic Applications. Mol. Pharm. 2017, 14, 2359–2367. [Google Scholar] [CrossRef]
- Ulett, G.C.; Webb, R.I.; Ulett, K.B.; Cui, X.; Benjamin, W.H.; Crowley, M.; Schembri, M.A. Group B Streptococcus (GBS) Urinary Tract Infection Involves Binding of GBS to Bladder Uroepithelium and Potent but GBS-Specific Induction of Interleukin 1α. J. Infect. Dis. 2010, 201, 866–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beydokthi, S.S.; Sendker, J.; Brandt, S.; Hensel, A. Traditionally used medicinal plants against uncomplicated urinary tract infections: Hexadecyl coumaric acid ester from the rhizomes of Agropyron repens (L.) P. Beauv. with antiadhesive activity against uropathogenic E. coli. Fitoterapia 2017, 117, 22–27. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.W.; Aebi, M.; Darvill, A.G.; Kinoshita, T.; Packer, N.H.; Prestegard, J.H.; et al. Essentials of Glycobiology, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015–2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK310274/ (accessed on 7 September 2021).
- Lamale, L.M.; Lutgendorf, S.K.; Zimmerman, M.B.; Kreder, K.J. Interleukine-6, Histamine, and Methyllhistamine as diagnostic markers for interstitial cystitis. Urology 2006, 68, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Killinger, K.; Tyagi, V.; Nirmal, J.; Chancellor, M.; Peters, K.M. Urinary Chemokines as Noninvasive Predictors of Ulcerative Interstitial Cystitis. J. Urol. 2012, 187, 2243–2248. [Google Scholar] [CrossRef] [Green Version]
- Hann-Chorng, K. Potential urine and serum biomarkers for patients with bladder pain syndrome/interstitial cystitis. Int. J. Urol. 2014, 21, 34–41. [Google Scholar] [CrossRef]
- Schulz, A.; Vestweber, A.-M.; Dressler, D. Anti-Inflammatory Action of a Hyaluronic Acid-Chondroitin Sulfate Preparation in an in vitro Bladder Model. Aktuel. Urol. 2009, 40, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Cvach, K.; Rosamilia, A. Review of intravesical therapies for bladder pain syndrome / interstitial cystitis. Transl. Androl. Urol. 2015, 4, 629–637. [Google Scholar] [CrossRef]
- Engelhardt, P.F.; Morakis, N.; Daha, L.K.; Esterbauer, B.; Riedl, C.R. Long-term results of intravesical hyaluronan therapy in bladder pain syndrome/interstitial cystitis. Int. Urogynecol. J. 2011, 22, 401–405. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruggeri, M.; Pavan, M.; Soato, M.; Panfilo, S.; Barbera, C.; Galesso, D.; Miele, D.; Rossi, S.; Di Lucia, A.; Ferrari, F.; et al. Synergy of Hydeal-D® and Hyaluronic Acid for Protecting and Restoring Urothelium: In Vitro Characterization. Pharmaceutics 2021, 13, 1450. https://doi.org/10.3390/pharmaceutics13091450
Ruggeri M, Pavan M, Soato M, Panfilo S, Barbera C, Galesso D, Miele D, Rossi S, Di Lucia A, Ferrari F, et al. Synergy of Hydeal-D® and Hyaluronic Acid for Protecting and Restoring Urothelium: In Vitro Characterization. Pharmaceutics. 2021; 13(9):1450. https://doi.org/10.3390/pharmaceutics13091450
Chicago/Turabian StyleRuggeri, Marco, Mauro Pavan, Matteo Soato, Susi Panfilo, Carlo Barbera, Devis Galesso, Dalila Miele, Silvia Rossi, Alba Di Lucia, Franca Ferrari, and et al. 2021. "Synergy of Hydeal-D® and Hyaluronic Acid for Protecting and Restoring Urothelium: In Vitro Characterization" Pharmaceutics 13, no. 9: 1450. https://doi.org/10.3390/pharmaceutics13091450