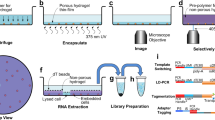
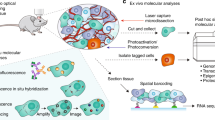
Abstract
The functional activity and differentiation potential of cells are determined by their interactions with surrounding cells. Approaches that allow unbiased characterization of cell states while at the same time providing spatial information are of major value to assess this environmental influence. However, most current techniques are hampered by a tradeoff between spatial resolution and cell profiling depth. Here, we develop a photocage-based technology that allows isolation and in-depth analysis of live cells from regions of interest in complex ex vivo systems, including primary human tissues. The use of a highly sensitive 4-nitrophenyl(benzofuran) cage coupled to a set of nanobodies allows high-resolution photo-uncaging of different cell types in areas of interest. Single-cell RNA-sequencing of spatially defined CD8+ T cells is used to exemplify the feasibility of identifying location-dependent cell states. The technology described here provides a valuable tool for the analysis of spatially defined cells in diverse biological systems, including clinical samples.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The processed single-cell RNA-sequencing data are deposited in the NCBI Gene Expression Omnibus (GEO) under accession no. GSE175813. Source data are provided with this paper.
Code availability
The code reproducing the analyses in this study is available as a supplementary file to NCBI GEO accession no. GSE175813.
References
Thommen, D. S. & Schumacher, T. N. T-cell dysfunction in cancer. Cancer Cell 33, 547–562 (2018).
Giesen, C. et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat. Methods 11, 417–422 (2014).
Keren, L. et al. MIBI-TOF: a multiplexed imaging platform relates cellular phenotypes and tissue structure. Sci. Adv. 5, eaax5851 (2019).
Goltsev, Y. et al. Deep profiling of mouse splenic architecture with CODEX multiplexed imaging. Cell 174, 968–981 (2018).
Medaglia, C. et al. Spatial reconstruction of immune niches by combining photoactivatable reporters and scRNA-seq. Science 358, 1622–1626 (2017).
Rodriques, S. G. et al. Slide-seq: a scalable technology for measuring genome-wide expression at high spatial resolution. Science 363, 1463–1467 (2019).
Moncada, R. et al. Integrating microarray-based spatial transcriptomics and single-cell RNA-seq reveals tissue architecture in pancreatic ductal adenocarcinomas. Nat. Biotechnol. 38, 333–342 (2020).
Vickovic, S. et al. High-definition spatial transcriptomics for in situ tissue profiling. Nat. Methods 16, 987–990 (2019).
Thrane, K., Eriksson, H., Maaskola, J., Hansson, J. & Lundeberg, J. Spatially resolved transcriptomics enables dissection of genetic heterogeneity in stage III cutaneous malignant melanoma. Cancer Res. 78, 5970–5979 (2018).
Ståhl, P. L. et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science 353, 78–82 (2016).
Berglund, E. et al. Spatial maps of prostate cancer transcriptomes reveal an unexplored landscape of heterogeneity. Nat. Commun. 9, 2419 (2018).
Maniatis, S. et al. Spatiotemporal dynamics of molecular pathology in amyotrophic lateral sclerosis. Science 364, 89–93 (2019).
Hu, K. H. et al. ZipSeq: barcoding for real-time mapping of single cell transcriptomes. Nat. Methods 17, 833–843 (2020).
Sanchez, E. & Huse, M. Spatial and temporal control of T-cell activation using a photoactivatable agonist. J. Vis. Exp. 2018, 56655 (2018).
Mayer, G. & Hechel, A. Biologically active molecules with a ‘light switch’. Angew. Chem. Int. Ed. 45, 4900–4921 (2006).
Klán, P. et al. Photoremovable protecting groups in chemistry and biology: reaction mechanisms and efficacy. Chem. Rev. 113, 119–191 (2013).
Yu, H., Li, J., Wu, D., Qiu, Z. & Zhang, Y. Chemistry and biological applications of photo-labile organic molecules. Chem. Soc. Rev. 39, 464–473 (2010).
Lee, H. M., Larson, D. R. & Lawrence, D. S. Illuminating the chemistry of life: design, synthesis and applications of ‘caged’ and related photoresponsive compounds. ACS Chem. Biol. 4, 409–427 (2009).
Icha, J., Weber, M., Waters, J. C. & Norden, C. Phototoxicity in live fluorescence microscopy, and how to avoid it. BioEssays 39, 1700003 (2017).
Lusic, H. & Deiters, A. A new photocaging group for aromatic N-heterocycles. Synthesis 2006, 2147–2150 (2006).
Young, D. D. & Deiters, A. Photochemical hammerhead ribozyme activation. Bioorg. Med. Chem. Lett. 16, 2658–2661 (2006).
Komori, N. et al. Design and synthesis of a new chromophore, 2-(4-nitrophenyl)benzofuran, for two-photon uncaging using near-IR light. Chem. Commun. 52, 331–334 (2016).
Maurits, E. et al. Immunoproteasome inhibitor–doxorubicin conjugates target multiple myeloma cells and release doxorubicin upon low-dose photon irradiation. J. Am. Chem. Soc. 142, 7250–7253 (2020).
Guimaraes, C. P. et al. Site-specific C-terminal and internal loop labeling of proteins using sortase-mediated reactions. Nat. Protoc. 8, 1787–1799 (2013).
Dorr, B. M., Ham, H. O., An, C., Chaikof, E. L. & Liu, D. R. Reprogramming the specificity of sortase enzymes. Proc. Natl Acad. Sci. USA 111, 13343–13348 (2014).
Capasso, S., Mazzarella, L., Sica, F., Zagari, A. & Salvadori, S. Spontaneous cyclization of the aspartic acid side chain to the succinimide derivative. J. Chem. Soc., Chem. Commun. 1992, 919–921 (1992).
Johnson, K. Y., Liu, L. & Vincent, T. S. Minimal FLAG sequence useful in the functional epitope tagging of H-ras. Biotechniques 32, 1270–1280 (2002).
Einhauer, A. & Jungbauer, A. The FLAGTM peptide, a versatile fusion tag for the purification of recombinant proteins. J. Biochem. Biophys. Methods 49, 455–465 (2001).
Vanier, G. S. Microwave-assisted solid-phase peptide synthesis based on the Fmoc protecting group strategy (CEM). Methods Mol. Biol. 1047, 235–249 (2013).
Bannas, P. et al. Molecular imaging of tumors with nanobodies and antibodies: timing and dosage are crucial factors for improved in vivo detection. Contrast Media Mol. Imaging 10, 367–378 (2015).
Jaitin, D. A. et al. Massively parallel single-cell RNA-seq for marker-free decomposition of tissues into cell types. Science 343, 776–779 (2014).
Baran, Y. et al. MetaCell: analysis of single-cell RNA-seq data using K-nn graph partitions. Genome Biol. 20, 206 (2019).
Ayers, M. et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Invest. 127, 2930–2940 (2017).
Russo, R. C., Garcia, C. C., Teixeira, M. M. & Amaral, F. A. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev. Clin. Immunol. 10, 593–619 (2014).
Repeke, C. E., Garlet, T. P., Fonseca, A. C., Silveira, E. M. & Garlet, G. P. in Encyclopedia of Signaling Molecules (ed. Choi, S.) 805–809 (Springer, 2018); https://doi.org/10.1007/978-3-319-67199-4_10
Ghorani, E. et al. The T-cell differentiation landscape is shaped by tumour mutations in lung cancer. Nat. Cancer 1, 546–561 (2020).
Van De Graaff, M. J. et al. Conditionally controlling human TLR2 activity via trans-cyclooctene caged ligands. Bioconjug. Chem. 31, 1685–1692 (2020).
Dieu-Nosjean, M. C., Goc, J., Giraldo, N. A., Sautès-Fridman, C. & Fridman, W. H. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 35, 571–580 (2014).
Antos, J. M. et al. Site-specific protein labeling via sortase-mediated transpeptidation. Curr. Protoc. Protein Sci. 89, 15.3.1–15.3.19 (2017).
Dijkgraaf, F. E. et al. Tissue patrol by resident memory CD8+ T cells in human skin. Nat. Immunol. 20, 756–764 (2019).
Bracke, M., Lammers, J. W. J., Coffer, P. J. & Koenderman, L. Cytokine-induced inside-out activation of FcαR (CD89) is mediated by a single serine residue (S263) in the intracellular domain of the receptor. Blood 97, 3478–3483 (2001).
Hoekstra, M. E. et al. Long-distance modulation of bystander tumor cells by CD8+ T-cell-secreted IFN-γ. Nat. Cancer 1, 291–301 (2020).
Van Rooij, N. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J. Clin. Oncol. 31, e439–e442 (2013).
Linnemann, C. et al. High-throughput identification of antigen-specific TCRs by TCR gene capture. Nat. Med. 19, 1534–1541 (2013).
Li, H. et al. Dysfunctional CD8 T cells form a proliferative, dynamically regulated compartment within human melanoma. Cell 176, 775–789 (2019).
Acknowledgements
Plasmid sequences for αCD8 nanobodies were kindly provided by 121Bio with support from M. Gostissa and G. Grotenbreg. We thank H. Ploegh for providing the sortase expression vector. We thank M. Marqvorsen, M. de Weert, M. de Bruijn, K. Bresser, D. Philips, D. Elatmioui, P. Hekking, N. Meeuwenoord, H. van den Elst, B. Florea, L. Bornes and staff of the NKI Flow Cytometry facility for technical support and input, and members of the van Kasteren, van Rheenen and Schumacher laboratories for discussions. This work was supported by ERC AdG SENSIT grant 742259 to T.N.S. and ERC StG Crosstag grant 639005 and ERC Cog KineTic grant 865175 to S.I.v.K.
Author information
Authors and Affiliations
Contributions
A.M.v.d.L., M.E.H., L.R., M.J.v.d.G., S.I.v.K. and T.N.S. conceived the project and contributed to experimental design. A.M.v.d.L., M.E.H., C.L.G.J.S. and M.T. designed, performed and analyzed the biological experiments. C.L.G.J.S. and L.Y.Y.C. performed microscopy experiments. L.R., M.T., M.J.v.d.G. and S.I.v.K. designed, synthesized and validated the photocaged compounds. L.R. and C.L.G.J.S. contributed equally to the execution of the experiments. H.L. performed single-cell sequencing and E.D. performed sequence alignments. A.M.v.d.L. performed computational analysis. H.L., A.B., Y.L. and A.T. provided input on the computational analysis. D.S.T. was responsible for human tumor sample acquisition. A.M.v.d.L., M.E.H., L.R., S.I.v.K. and T.N.S. wrote the manuscript, with input from all other co-authors. J.v.R., I.A., S.I.v.K. and T.N.S. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemical Biology thanks Johannes Broichhagen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Synthesis of bis functionalized NPBF based photocleavable linker and the photo-sensitive tag (PsT).
(a) Reagents and conditions for synthesis of the NPBF cage: a) bromoacetaldehyde diethyl acetal, KOH, NMP, 70 oC, 15 hours, 90%; b) polyphosphoric acid, toluene, 111 oC, 16 hours, 33%; c) BBr3, DCM, 1 hour at - 78 oC then 1 hour at room temperature, 89%; d) TBDMSCl, imidazole, DMF, 1 hour, room temperature, 70%; e) (i) n-BuLi, THF, -78 oC, 1 hour, (ii) triisopropyl borate, 30 minutes at -78 oC then 30 minutes at room temperature; f) Pd(PPh3)4, K2CO3, THF/H2O, 75 oC, 18 hours, 75%; g) HF-pyridine, THF, room temperature, 1 hour, 77%; h) K2CO3, propargyl bromide, room temperature, 5 hours, 86%; i) NaBH4, 1,4-dioxane/methanol, room temperature, 1 hour, 0 oC to room temperature, 88%; j) N,N’-disuccinimidyl carbonate, Et3N, DMF, room temperature, 4 hours, 79%. (b) Reagents and conditions for synthesis of the PsT: a) 1% TFA, DCM, room temperature, 30 minutes (2x); b) 1, DiPEA, DMF, room temperature, 18 hours; c) 20% piperidine, DMF, room temperature, 2 minutes (3x), d) TFA, TIS, H2O 95/2.5/2.5, room temperature, 3 hours; e) CuSO4, sodium ascorbate, THPTA, AF594-N3, H2O, t-BuOH room temperature, 2 hours. (c) Uncaging efficiency of the PsT over time. 10µM PsT (in water) was uncaged with 420-nm light using a 1W LED-lamp for the indicated times and reaction products were measured using LC-MS analysis. Data points represent the mean +/- s.d. of technical triplicates (n=3) from a single experiment.
Extended Data Fig. 2 Generation of stable dimeric αCD8 nanobodies.
(a) Binding stability of αCD8 nanobodies to CD8+ T cells. Top panel: a first population of CD8+ T cells was stained with FITC labeled monomeric nanobody (αCD8M-FITC) and a second population was stained with αCD8M-AF647. Bottom panel: a first population of CD8+ T cells was stained with FITC labeled dimeric nanobody containing recognition domains that are identical to the αCD8M (αCD8D−2-FITC) and a second population was stained with αCD8D−2-AF647. Subsequently, two cell populations labeled with either monomeric or dimeric αCD8 reagents were mixed, incubated at 37oC, and exchange of αCD8 nanobodies was measured by flow cytometry. Quantification of this αCD8D−2 clone and of a second nanobody clone αCD8D−1 is depicted in Extended data Fig. 2b. (b) Data depict AF647 fluorescence signal of two CD8+ T cell populations that were either stained with the indicated αCD8D-AF647 or with the corresponding αCD8D-FITC, and that were mixed and subsequently incubated at 37 °C. Note the lack of substantial AF647 signal on the FITC-labeled bystander pool. Data are representative of 2 independent experiments.
Extended Data Fig. 3 αCD8-PsT nanobody generation and recognition by αFLAG antibodies.
(a) Coomassie staining of sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel showing the indicated nanobodies before and after labeling with the PsT, FITC or AF647. Band marked with the open triangle represents the PsT-labeled αCD8D−1 and band marked with the closed triangle represents a hydrolysis product, that has previously been described in C-terminal protein labeling39. Data obtained from a single experiment. (b) Binding of αFLAG antibodies to the FLAG-tag with or without the NPBF cage (αCD8D−1-PsT and αCD8D−1-T respectively). Cells labeled with αCD8D−1-PsT (left panel) or αCD8D−1-T (right panel) were stained with polyclonal αFLAG antibodies or αFLAG clone L5. Data obtained from a single experiment. (c) Specific binding of αFLAG antibody clone D6W5B to the uncaged FLAG-tag. Cells stained with αCD8D−1 nanobody conjugated to either the FLAG-tag without cage (αCD8D−1-T, upper panel) or the FLAG-tag containing the NPBF cage (αCD8D−1-PsT, bottom panel) were stained with αFLAG antibody clone D6W5B. Note that appreciable AF488 signal is only observed when the uncaged FLAG-tag is used. Representative data from 3 independent experiments are depicted.
Extended Data Fig. 4 Flow cytometry gating strategy to distinguish uncaged and caged CD8+ T cells.
Data depict gating strategy used for flow cytometry analysis as well as for index sorting of uncaged CD8+ T cells. Viable single CD8+ T cells are selected based on double staining with αCD8D−1-PsT and αCD8D−2-FITC (serving as a stable signal to identify cells after loss of the AF594-positive cage). Uncaged cells are distinguished from caged cells based on their elevated αFLAG signal and reduced αCD8D−1-PsT signal (AF594low αFLAGhigh).
Extended Data Fig. 5 Characterization of nanobodies labeled with monomeric or dimeric PsT.
(a) SDS-PAGE analysis of nanobody-PsT generated under non-reducing or reducing conditions. Left panel: Coomassie stained SDS-PAGE gel showing the reduced (left) or non-reduced (right) versions of PsT-labeled αCD8D−1, αCD47D and αHLA-ID nanobodies. Right panel: AF594 fluorescence signal of the same gel. Note the increased size of PsT-nanobodies when coupled under non-reducing conditions as compared to PsT-nanobodies generated under reducing condition, indicating the presence of PsT dimers under non-reducing conditions (see also LC-MS and NMR spectra in Supplementary Information). Lower band in both conditions represents the non-fluorescent hydrolysis product that is formed during sortase reactions39. Data obtained from a single experiment. (b) Flow cytometric analysis of the uncaging of CD8+ T cells labeled with αCD8D−1-PsT nanobodies generated under reducing or non-reducing conditions (n = 2 technical replicates). Data were obtained from a single experiment.
Extended Data Fig. 6 Quantification of uncaging efficiency.
(a) Flow cytometric analysis of αCD8D−1-PsT-labeled CD8+ T cells upon exposure to the indicated laser intensities or 1 hour to natural light. (b) Viability of uncaged (AF594low αFLAGhigh) or caged (AF594high αFLAGlow) CD8+ T cells from samples exposed to the indicated laser intensities, as measured by flow cytometric analysis following IR-dye live-dead staining. Data shown are obtained from a single experiment. (c) Correlation between uncaged surface area and the observed fraction uncaged αCD8D−1-PsT labeled CD8+ T cells in peripheral blood mononuclear cells (PBMCs). 0%, 50%, or 100% of the surface area of wells containing αCD8D−1-PsT labeled cells was uncaged, and samples were analyzed by flow cytometry. (d) Quantification of the correlation between uncaged surface area and fraction uncaged CD8+ T cells as measured with flow cytometry, as shown in Extended data Fig. 6c. After uncaging of the indicated surface areas, cells were mixed at a 1:1 ratio with PBMCs from non-exposed samples. Cells from exposed and non-exposed samples were distinguished by labeling samples with different αCD3 antibodies before mixing. Blue line represents the fraction of uncaged (AF594low αFLAGhigh) cells after local exposure with 405-nm light. Green line represents the fraction of AF594low αFLAGhigh cells within the second sample that was not exposed to 405-nm (that is not uncaged). Note that αCD8D−1-PsT binding is stable throughout the experimental pipeline, as demonstrated by the absence of AF594low αFLAGhigh cells in the non-exposed sample. Line graphs show mean of technical triplicates from one experiment. Data are presented as mean values ± s.d.
Extended Data Fig. 7 Single cell mRNA sequencing of locally activated CD8+ T cells.
(a) 2D projection of the 9,237 cells as shown in Fig. 4a divided into 99 metacells. Single cells are depicted as dots. Metacells are uniquely numbered and depicted as circles. Lines indicate relatedness between metacells. (b) Confusion matrix of all metacells. Clusters indicate groups of related metacells and are defined as main cell groups as shown by the color code below the confusion matrix that corresponds to the color code used in Fig. 4a and Extended data Fig. 7a. (c) Quantification of T cell states of uncaged (AF594low αFLAGhigh) CD8+ T cell populations from control samples that only contained either Ag+ or Ag− tumor cells, and in which an area was uncaged that is comparable to the areas uncaged in the tumor island conditions. Tumor cells are excluded from the analysis. Data are representative of 2 independent experiments. (d) αFLAG and αCD8D−1-PsT signals, as acquired during index sorting. αFLAG and αCD8D−1-PsT signals are depicted for those cells in the gene expression dataset that are derived from tumor island cultures in which either CD8+ T cells in an Ag+ area (left panel) or in an Ag− area (middle panel) were uncaged, or from the control condition (right panel) in which no cells were uncaged. Line depicts the cut-off between caged and uncaged CD8+ T cells. (e) Quantification of T cell states for all technical replicates of uncaged (AF594low αFLAGhigh) CD8+ T cells from antigen positive (Ag+, 193–230 cells) and antigen negative (Ag−, 186–232 cells) tumor cell areas. The three replicates jointly underlie the data in Fig. 4c. Tumor cells are excluded from the analysis. Representative data of 2 independent experiments. (f) Bar plots depicting the top 30 genes of which expression correlates most strongly with IFNG expression, together defining the IFNG module. (g) Bar plots depicting the top 30 genes of which expression correlates most strongly with the two alternative anchor genes CCL4 (left) and CXCL8 (right). (h) Gene expression enrichment in uncaged (AF594low αFLAGhigh) CD8+ T cells from Ag+ versus Ag− areas. Bar plot depicts the top 30 genes showing increased expression in uncaged CD8+ T cells from an Ag+ area as compared to uncaged CD8+ T cells from an Ag− area. Gene enrichments are based on data from a single experiment. (i) Correlation of variable genes between uncaged CD8+ T cells from Ag+ versus Ag− areas in independent experiments. Scatterplot shows the gene enrichment scores in AF594low αFLAGhigh CD8+ T cells from samples in which Ag+ versus Ag− areas were uncaged for 2 independent experiments.
Extended Data Fig. 8 Cell states from CD8+ T cells residing in intermingled tumor areas.
(a) Confocal image of coculture of Ag-specific CD8+ T cells with tumor cell islands in which Ag+ tumor cell areas (green) and Ag− tumor cell areas (magenta) are intermingled. Scale bar represents 2 mm. Representative of 2 independent experiments. (b) Quantification of T cell states in uncaged αCD8D−1-PsT labeled CD8+ T cells from Ag+ and Ag− areas in a mixed tumor culture as shown in Extended data Fig. 8a. Bar plots show the quantification of T cell states as defined in Fig. 4a of uncaged CD8+ T cell populations from samples in which either Ag+ or Ag− areas were uncaged (252 and 236 cells, respectively). Tumor cells are excluded from the analysis. Two-tailed Chi-square test was performed (**** indicates p < 1*10−15). Data shown were obtained in a single experiment.
Extended data Fig. 9 Multiplexed uncaging of cells in different areas.
(a) Schematic representation of multiplexed analysis of cells in adjacent areas. (b) Representative confocal image of OVCAR5 tumor cell culture consisting of adjacent islands of GFP+ cells (population 1) and GFP− cells stained for expression of a cell membrane marker (population 2). Scale bar represents 250μm. Enlarged areas are indicated by the boxes. Tumor cells in a first island (area A, marked with the dashed white line), containing cells from population 1, were uncaged in a first uncaging round and in situ stained with a first fluorescently labeled αFLAG antibody (αFLAG1). Subsequently, tumor cells in a second island in the same culture (area B, marked by the dashed yellow line), containing cells from population 2, were uncaged in a second round of uncaging. After staining with a second fluorescent αFLAG antibody (αFLAG2), flow cytometric analysis was performed. Images are representative of 2 independent experiments. (c) Bar plot shows the fraction of population 1 (GFP+) and population 2 (membrane-stained) cells within the AF594low and αFLAG1−high (that is uncaged in round 1) population, or within the AF594low and αFLAG2−high (that is uncaged in round 2) population (n = 3 technical replicates). Data are representative of 2 independent experiments.
Supplementary information
Supplementary Information
Supplementary Note and Data 2
Supplementary Data 1
Metadata of cells analyzed with single-cell RNA sequencing.
Supplementary Data 3
Genesets related to strong transcriptional modules that were excluded from analysis.
Supplementary Data 4
Genesets related to strong transcriptional modules (lateral genes).
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed gel.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed gels.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
About this article
Cite this article
van der Leun, A.M., Hoekstra, M.E., Reinalda, L. et al. Single-cell analysis of regions of interest (SCARI) using a photosensitive tag. Nat Chem Biol 17, 1139–1147 (2021). https://doi.org/10.1038/s41589-021-00839-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-021-00839-x
This article is cited by
-
Photoselective sequencing: microscopically guided genomic measurements with subcellular resolution
Nature Methods (2023)
-
Bridging live-cell imaging and next-generation cancer treatment
Nature Reviews Cancer (2023)
-
Change can be SCARI
Nature Chemical Biology (2021)