Abstract
Background
Magnesium-based bioresorbable Magmaris stents are rapidly resorbed. Few randomized studies have evaluated the efficacy of such stents in patients with acute coronary syndrome.
Aim
To investigate late lumen loss as assessed via quantitative coronary angiography (QCA) and optical coherence tomography (OCT) in patients with acute coronary syndrome treated with Magmaris stents or permanent, everolimus-eluting metallic Xience stents.
Methods and Results
This PRAGUE-22 study was a two-centre, investigator-initiated, randomized study. Fifty patients were randomized based on the inclusion criteria for acute coronary syndrome and the anatomical suitability to receive Magmaris or Xience stents. The patient characteristics did not differ between the Magmaris group (n = 25) and Xience group (n = 25). The mean ages were 57.0 ± 10.5 vs. 55.5 ± 9.2 years (p = 0.541) and the total implanted stent length was 24.6 ± 10.7 mm vs. 27.6 ± 11.1 mm (p = 0.368), respectively. Four clinical events occurred in the Magmaris group and one in the Xience group during 12 months of follow-up. The extent of late lumen loss (assessed via QCA) at 12 months was greater in the Magmaris group than in the Xience group (0.54 ± 0.70 vs. 0.11 ± 0.37 mm; p = 0.029). The late lumen loss diameter (measured via OCT) in the Magmaris group was also significantly larger than that in the Xience group (0.59 ± 0.37 vs. 0.22 ± 0.20 mm; p = 0.01).
Conclusion
Implantation of a magnesium-based bioresorbable stent in patients with acute coronary syndrome is associated with a greater extent of late lumen loss at the 12-month follow-up compared with implantation of a permanent, everolimus-eluting metallic stent.
Trial Registration: ISRCTN89434356
Similar content being viewed by others
Introduction
In 2011, interventional cardiologists enthusiastically welcomed the first generation of polymeric bioresorbable stents. However, the Absorb stent (Abbott Vascular, Santa Clara, CA, USA) was associated with a higher risk of target lesion revascularization and stent thrombosis compared with drug-eluting metallic stents in randomized trials [1, 2] and in an acute myocardial infarction setting [3]. Furthermore, resorption only took place more than 3 years after implantation, being complete at 5 years [4, 5]. Magnesium-based bioresorbable stents represent different technology with quicker resorption process and are sometimes viewed as second-generation bioresorbable stents. Early studies reported good clinical results for up to 3 years after implantation in patients with stable coronary artery disease (simple lesions) [6, 7]. However, the first randomized study in patients with ST-elevation acute myocardial infarctions reported a lower angiographic efficacy and a higher rate of target lesion failure (TLF) after the implantation of magnesium-based bioresorbable stents compared to the implantation of sirolimus-eluting metallic stents at the 12-month follow-up [8]. We investigated the 12-month efficacy of bioresorbable, magnesium-based sirolimus-eluting stents compared to everolimus-eluting metallic stents in patients with acute coronary syndrome.
Methods
This was a two-centre, investigator-initiated academic randomized study. Patients were randomized using the envelope method between May 2017 and December 2019 into Magmaris stent- (Biotronic AG, Bulach, Switzerland) and Xience stent-treated (Abbott, Santa Clara, CA, USA) groups. The inclusion criteria were ST-elevation myocardial infarction (STEMI) within 24 h of symptom onset, non-ST elevation myocardial infarction (non-STEMI), or unstable angina caused by thrombotic acute coronary stenosis, and a coronary artery diameter appropriate for implantation of either stent (vessel diameter 2.7 to 3.7 mm). The exclusion criteria were cardiogenic shock, pulmonary oedema, expected survival < 3 years because of severe comorbidities, any contraindication for 12 months of dual antiplatelet treatment (including peroral anticoagulants), any diffuse calcification or extreme tortuosity of the target vessel, in-stent restenosis or stent thrombosis as the culprit lesion, and left main vessel stenosis. The study was approved by the ethics committee of each centre and by our national multicentric ethics committee, and written informed consent was obtained from all patients. The study adhered to the tenets of the Declaration of Helsinki.
Procedural Technique
The treating physicians had considerable experience with bioresorbable stents and adhered to a standardized technique that accounted for stenosis predilation using a balloon of diameter 0.5 mm or less than that of the vessel and confirmed the correct sizing and postdilation of the implanted stent using a noncompliant balloon inflated to > 16 atmospheres of the same nominal size as the scaffold implantation balloon or up to 0.5 mm larger. The implantation of metallic Xience stents was left to the discretion of the physicians, with postdilation strongly recommended. Optical coherence tomography (OCT) of acute-phase patients was recommended but not mandatory (the most common reasons for omission were haemodynamic instability, clinically significant arrhythmia, and lack of an available OCT catheter). The use of the same type of stent was recommended if a second (nontarget) lesion were to be treated.
Follow-up and Study Endpoints
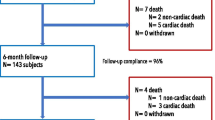
The protocol mandated dual antiplatelet therapy for 12 months after the implantation of either stent. All patients were examined at 1 and 6 months by blinded cardiologists. Repeat quantitative coronary angiography (QCA) and OCT were performed at 12 months. Figure 1 shows the numbers of patients who underwent clinical follow-up and coronary artery imaging at 12 months. The primary endpoints of our study were late lumen loss evident at the 12-month follow-up as assessed by QCA and OCT. The secondary endpoints were the device and procedural success rates, combined clinical endpoints (death, stent thrombosis, target vessel myocardial infarction), clinical TLF rate, Magmaris stent resorption status, and qualitative assessment of healing in both groups.
QCA
The treated segments (5 mm proximal and distal to the scaffold edge) were analyzed using Philips IntelliSpace cardiovascular software (Philips Medical Systems, Eindhoven, the Netherlands). Image calibration was performed using a contrast-filled guiding catheter. The following QCA parameters were measured after stent implantation in both groups: proximal and distal reference vessel diameters (RVDs)—defined as those of the largest lumina within 5 mm of the proximal and distal edges, the minimal lumen diameters (MLDs) within the scaffolds, and the mean scaffold diameters. The stenosis diameter was calculated from the MLD, and the average of the proximal and distal RVDs and was expressed as a percentage. The extent of late lumen loss was represented as the MLD at baseline minus the MLD at follow-up. Binary restenosis was defined as a stenosis diameter > 50% on follow-up QCA.
OCT
OCT of infarct-related vessels with stents was performed using the frequency-domain C7 system and a Dragonfly or Dragonfly Duo catheter (St. Jude Medical, St. Paul, MN, USA). The pullback speed was 20 mm/s, and the image acquisition rate was 100 or 200 frames/s. OCT measurements were performed using commercial software for offline analysis (Ilumien Optis system; LightLab Imaging, Westford, MA, USA). The OCT results were analyzed on a per-patient and per-frame basis. The reference vessel area and diameter were measured at the sites with the largest lumina within 5 mm of the proximal and distal scaffold edges. Stent area/diameter and lumen area/diameter at follow-up were measured using vessel cross-sections at 1-mm intervals. The mean stent/lumen area and diameter were defined as the means of those of all cross-sections within the device. The minimal luminal area was the smallest area within the device. QCA and OCT were performed by a single experienced unblinded operator (PT); qualitative OCT assessment of healing and neoatherosclerosis was conducted by two operators working in consensus. For qualitative analysis, we used the recent methodology of Gomez-Lara [9]. Depending on the highest numbers of cross-sections exhibiting one of four possible bioresorption/healing profiles, the outcomes of using both devices were classified into the following groups: struts indiscernible, visible struts completely integrated into the vessel wall, visible struts protruding into the lumen (causing characteristic bumps), and visibly protruding struts malapposed to the vessel wall. Neoatherosclerosis apparent on follow-up OCT was defined as the presence of at least one of the following findings between the stent and lumen or < 200 μm from the end-luminal border when struts were indiscernible: a fibrocalcific plaque (a signal-poor region with sharply delineated upper and lower borders), lipid-rich plaques (diffusely bordered, signal-poor regions), or signs of neovascularization [10].
Statistical Analysis
Standard descriptive statistics were use; categorical variables are presented as absolute values and relative frequencies, whereas continuous variables are described as means with standard deviations. The significance of between-group differences was computed using Fisher’s exact test for two categorical variables, or the maximum likelihood χ2 test for variables with more than two categories. The significance of changes in continuous variables (QCA and OCT data) between baseline and follow-up was evaluated using the paired t-test. P-values < 0.05 were considered to indicate statistical significance. All analyses were performed with the aid of SPSS ver. 26.0.0.0 (IBM Corp., Armonk, NY, USA).
Results
Fifty patients were enrolled; 25 patients were initially assigned to each group. There was one crossover from the Magmaris group, as a stent of the required length was not available and a Xience stent was thus placed. Table 1 summarizes the patient and procedural characteristics. There were no significant between-group differences with the exceptions of the lower lesion predilation and stent postdilation rates in the control group. Twelve-month clinical follow-up data were available for all 50 patients, and 15 patients in the Magmaris group and 21 in the control group agreed to undergo repeat QCA and OCT.
QCA Results
The baseline and follow-up QCA characteristics of patients in both groups are summarized in Table 2. There were no differences in the baseline MLD and baseline mean in-stent lumen diameter between the groups. At 12 months, the late lumen loss was larger in the Magmaris group than that in the control group (0.54 ± 0.70 vs. 0.11 ± 0.37 mm; p = 0.029). The stented segment stenosis increased significantly in the Magmaris group during follow-up based on the baseline and 12-month QCA results (7.00 ± 8.41% vs. 27.05 ± 20.58%; p < 0.001) but not in the Xience group (10.16 ± 8.73% vs. 15.52 ± 11.62%; p = 0.141).
OCT Results
One OCT dataset in the Magmaris group could not be subjected to final analysis because the image quality was poor. Also, four patients in the Magmaris group were excluded from the 12-month analysis because of TLF re-interventions featuring the implantation of permanent, drug-eluting metallic stents. Ultimately, 12-month follow-up OCT data on 14 patients in the Magmaris group and 21 patients in the Xience group were available. Of these, all 14 patients in the Magmaris group and 9 patients in the Xience group had undergone baseline OCT after stent implantation. Table 3 compares the OCT measurements between the groups at the 12-month follow-up, and the baseline and follow-up measurements of each group. The late lumen loss diameter was significantly larger in the Magmaris group compared to the Xience group (0.59 ± 0.37 vs. 0.22 ± 0.20 mm; p = 0.01). Qualitative assessment at the 12-month follow-up revealed that struts were indiscernible in 9 (60%) patients in the Magmaris group, visible struts were completely integrated into the vessel wall in 3 (20%) patients in the Magmaris group and 17 (81%) in the control group, and visible struts protruding into the lumen were apparent in 3 (20%) patients in the Magmaris group and 3 (14%) in the control group. Protruding malapposed struts were observed in one control patient but no Magmaris patients. Table 4 lists the quantitative OCT parameters for both groups at follow-up and for patients who underwent OCT at both baseline and follow-up (enabling comparison of the paired measurements, as with the QCA data above). Neoatherosclerosis was apparent in 7 of 15 (47%) patients in the Magmaris group and 10 of 21 (48%) patients in the control group.
Imaging Data from Patients Experiencing Clinical Events
During follow-up, three clinical events (all unstable angina) that required target lesion re-intervention and one stent thrombosis (associated with dual antiplatelet therapy discontinuation) occurred in the Magmaris group. One clinical event (unstable angina) that required target lesion re-intervention occurred in the control group. Table 4 shows the details. Patients with clinical events were treated with either ticagrelor or prasugrel, except for the patient with a stent thrombosis who had stopped taking clopidogrel 14 days before the event. The thrombosis was treated via thrombus aspiration and drug-eluting stent implantation. OCT was not performed during this procedure. Two patients in the Magmaris group exhibited early recurrence of angina symptoms and underwent coronary angiography; in-stent re-stenoses were apparent. Stent collapse with strut malpositioning and discontinuities were found on OCT of one patient in the Magmaris group (Fig. 2), who was then treated with a drug-eluting metallic stent implantation. Neoproliferation and stent collapse was seen in one patient in the Magmaris group who developed unstable angina symptoms at 10 months (Fig. 3). High-level stent-edge neoproliferation without stent collapse was observed in one control patient with target lesion failure (TLF).
Angiographic and OCT images obtained after percutaneous cardiac intervention in a patient with a Magmaris stent (left) and target lesion failure at 10 months after the procedure (right). Tight angiographic restenosis is evident in the images on the right. OCT performed after balloon predilation of the stenosis revealed neoproliferation and stent collapse without malpositioning
Discussion
We evaluated the utility of a magnesium-based bioresorbable stent in the setting of acute coronary syndrome. Our principal findings were that the extent of late lumen loss at the 12-month follow-up was significantly greater in the Magmaris group, complete resorption at 12 months postimplantation was evident in 60% of the Magmaris patients, and clinical events seemed to be associated with strut collapse in the Magmaris group.
Greater late lumen loss was experienced by the Magmaris group despite optimal implantation featuring high rates of both pre- and postdilation. Acute Magmaris stent recoil was not in play. The baseline angiographic data revealed similar acute residual stenosis rates of 7% in the Magmaris group and 10% in the control group. Furthermore, optimal final results after Magmaris implantation were confirmed via OCT in 23 of 25 patients. Similar findings in terms of late lumen loss were noted after Magmaris-stent use in the MAGSTEMI trial [8].
We noted advanced bioresorption at 12 months in approximately two-thirds of the patients with Magmaris stents, as did the OCT MAGSTEMI substudy [9]. Advanced bioresorption was unfortunately associated with vessel recoil in some segments and vessel remodelling of other segments. Neoatherosclerosis (fibrocalcified plaques near vessel lumina) may reflect preexisting atherosclerotic processes that became more visible after stent bioresorption. In both groups, the neoatherosclerotic changes mostly involved mildly fibrocalcified plaques.
In terms of clinical events, one case of stent thrombosis was probably attributable to antiplatelet medication nonadherence. Also, although data on the Magmaris stent are fewer than those for the Absorb stent, it appears that thrombosis is not a major issue with the Magmaris stent. This was confirmed in the Biosolve-IV trial; definitive/probable Magmaris stent-associated thromboses occurred in only 5 of 1075 (0.5%) patients [6]. Based on our imaging data and in vitro studies of two bioresorbable stents, we hypothesize that the high, acute radial force of the Magmaris stent better embeds the struts in vessel walls [11, 12]. However, the fast resorption and short length of scaffolding trigger more late lumen loss. These mechanisms also probably explain the stent collapses in patients with early clinical events. Our TLF rate after Magmaris-stent implantation was very similar to that of the MAGSTEMI trial (16.2% of the patients required target lesion revascularization). However, our TLF rate was almost threefold that of the BIOSOLVE-IV trial. Such discrepancies support the current European Society of Cardiology recommendation that bioresorbable stents should not be used other than in well-controlled clinical studies [13].
Limitations
The major limitations of our study are the small number of patients, the lack of calculation of a required sample size, and the incomplete imaging follow-up at 12 months (only baseline OCT data were available for some patients). However, most studies on Magmaris stenting in acute coronary settings also included a limited number of patients (the largest study, the MAGSTEMI trial, enrolled 150), and invasive follow-up data are seldom reported [14, 15]. Furthermore, at the time of study commencement, no data on imaging assessment of Magmaris stents in the acute coronary setting were available; thus, we could not calculate the required sample size. Our work should be viewed as a pilot randomized study that precedes future adequately powered studies.
Conclusion
Both QCA and OCT revealed a greater extent of late lumen loss in the Magmaris group than in the Xience group. Advanced bioresorption and the short length of scaffolding explain the strut collapses observed in patients with clinical events. Our data confirm the results of the MAGSTEMI trial that angiographic efficacy was lower after Magmaris stent than drug-eluting metallic stent placement in patients with acute coronary syndrome.
Impact on Daily Clinical Practice
Magnesium-based bioresorbable stents are not appropriate for routine clinical use in acute coronary settings. The extent of late lumen loss is considerable despite optimal implantation. Further improvements are needed.
Data Availability
The data that support the findings of this study are not openly available because they are human data but are available from the corresponding author upon reasonable request (author location: University Hospital Kralovske Vinohrady, Prague, Czech Republic).
References
Ali ZA, Gao R, Kimura T, et al. Three-year outcomes with the Absorb bioresorbable scaffold: individual-patient-data meta-analysis from the ABSORB randomized trials. Circulation. 2018;137(5):464–79.
Smits PC, Chang CC, Chevalier B, et al. Bioresorbable vascular scaffold versus metallic drug-eluting stent in patients at high risk of restenosis: The COMPARE-ABSORB randomised clinical trial. EuroIntervention. 2020;16(8):645–53.
Brugaletta S, Gori T, Tousek P, et al. Bioresorbable vascular scaffolds versus everolimus-eluting metallic stents in patients with ST-segment elevation myocardial infarction: 5-year results of the BVS-EXAMINATION study. EuroIntervention. 2020;15(16):1436–43.
Tousek P, Kocka V, Maly M, et al. Long-term follow-up after bioresorbable vascular scaffold implantation in STEMI patients: PRAGUE-19 study update. EuroIntervention. 2016;12(1):23–9.
Kocka V, Tousek P, Kozel M, et al. Bioresorbable scaffold implantation in STEMI patients: 5 years imaging subanalysis of PRAGUE-19 study. J Transl Med. 2020;18(1):33.
Verheye S, Wlodarczak A, Montorsi P, et al. BIOSOLVE-IV-registry: safety and performance of the Magmaris scaffold: 12-month outcomes of the first cohort of 1,075 patients. Catheter Cardiovasc Interv. 2021;98(1):E1–8.
Haude M, Ince H, Kische S, et al. Sustained safety and performance of the second-generation sirolimus-eluting absorbable metal scaffold: pooled outcomes of the BIOSOLVE-II and -III trials at 3 years. Cardiovasc Revasc Med. 2020;21(9):1150–4.
Sabate M, Alfonso F, Cequier A, et al. Magnesium-based resorbable scaffold versus permanent metallic sirolimus-eluting stent in patients with ST-segment elevation myocardial infarction: the MAGSTEMI Randomized Clinical Trial. Circulation. 2019;140(23):1904–16.
Gomez-Lara J, Ortega-Paz L, Brugaletta S, et al. Bioresorbable scaffolds versus permanent sirolimus-eluting stents in patients with ST-segment elevation myocardial infarction: vascular healing outcomes from the MAGSTEMI trial. EuroIntervention. 2020;16(11):e913–21.
Ortega-Paz L, Brugaletta B, Gomez-Lara J, et al. Target lesion revascularisation of bioresorbable metal scaffolds: a case series study and literature review. EuroIntervention. 2021;16(13):1100–3.
Cerrato E, Barbero U, Gil Romero JA, et al. Magmaris resorbable magnesium scaffold: state-of-art review. Future Cardiol. 2019;15(4):267–79.
Schmidt W, Behrens P, Brandt-Wunderlich C, et al. In vitro performance investigation of bioresorbable scaffolds - standard tests for vascular stents and beyond. Cardiovasc Revasc Med. 2016;17(6):375–83.
Neumann F-J, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165.
Ielasi A, Cerrato E, Geraci S, et al. Sirolimus-eluting magnesium resorbable scaffold implantation in patients with acute myocardial infarction. Cardiology. 2019;142(2):93–6.
Wlodarczak A, Lanocha M, Jastrzebski A, et al. Early outcome of magnesium bioresorbable scaffold implantation in acute coronary syndrome-the initial report from the Magmaris-ACS registry. Catheter Cardiovasc Interv. 2019;93(5):E287–92.
Acknowledgements
We would like to acknowledge Vojtech Novotny, MD., Ph.D., for helping with data collection.
Funding
The study was supported by the Charles University research programmes UNCE MED 002 and PROGRES Q38 and the INTERCARDIS project (EU no. CZ.02.1.01/0.0/0.0/16_026/0008388).
Author information
Authors and Affiliations
Contributions
PT and VK developed the study protocol and initiated the study in the University Hospital Kralovské Vinohrady. TL initiated the study in the cardiology department of Pardubice Hospital. PT, VK, IV, and TL collected data; PT and TL analyzed data. MN was responsible for the database and study co-ordination. MN analyzed data and performed the statistical analysis. PT, TL, and VK wrote the article; IV reviewed the manuscript.
Corresponding author
Ethics declarations
Research Involving Human Participants and/or Animals
The study was approved by the multicentre ethics committee of University Hospital Kralovske Vinohrady, Prague, Czech Republic (approval no. EK-VP/29/2017).
Informed Consent
All patients provided written informed consent.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Toušek, P., Lazarák, T., Varvařovský, I. et al. Comparison of a Bioresorbable, Magnesium-Based Sirolimus-Eluting Stent with a Permanent, Everolimus-Eluting Metallic Stent for Treating Patients with Acute Coronary Syndrome: the PRAGUE-22 Study. Cardiovasc Drugs Ther 36, 1129–1136 (2022). https://doi.org/10.1007/s10557-021-07258-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-021-07258-z