Abstract
Occurrence of stem rust, caused by Puccinia graminis f. sp. avenae, on oat fields in Europe may lead to significant yield losses. The last P. graminis virulence research in this region had been carried out in the years 1988 to 1996, therefore the aim of this study was the update of pathogen’s virulence structure as well as the evaluation of Pg race-specific resistance genes and their potential for use in practical breeding in central and eastern European growing conditions. To assess the pathotype structure of P. graminis in Poland and evaluate the potential of Pg resistance genes, 148 isolates were collected during 2017–2020. Their virulence structure was determined in laboratory conditions on 12 Pg differentials as well as oat lines ‘Pg-a’, ‘Alpha’, ‘Omega’ and ‘Wisconsin X-1588-2’. In total, 57 pathotypes were detected, of which the most dominant race was SSK. High frequency was also recorded for TSK, TKK and TTK. Puccinia graminis isolates were able to overcome from 5 to 11 Pg resistance genes with an average virulence complexity of 8.6. There was no isolate virulent to Pg12, ‘Pg-a’, ‘Alpha’ and ‘Omega’, hence these genes can be used in the breeding programmes. Gene Pg10 was also relatively effective, overcome by 33 out of 148 P. graminis isolates. The virulence profile observed may be representative of eastern Europe and beyond.
Similar content being viewed by others
Introduction
Stem rust is caused by Puccinia graminis (Pers.), an obligate biotrophic fungus capable of infecting over 365 species of cereals and grasses in 54 genera. Symptoms of stem rust include reddish-brown pustules containing urediniospores that develop mainly on stems and leaf sheaths but may occur on leaf blades and glumes (Leonard & Szabo, 2005). Stem rust has been a serious disease of the most frequently sown cereals in the world such as wheat, barley, rye and oat. Cultivated oat (Avena sativa L.) is attacked by Puccinia graminis Pers. f. sp. avenae Eriks. & E. Henn. leading to significant yield losses. Stem rust is especially dangerous when it attacks in the early stages of plant development. Disease affects plant foliage, lowers the straw quality, increases lodging and impedes harvesting. If infection occurs at the end of plant maturation, i.e. in the stage of wax ripeness, grain losses are less substantial (Roelfs et al., 1992).
The ability of P. graminis to reproduce sexually and asexually enables adaptation to host resistance changes. The fungus overwinters in the form of two-celled teliospores. In the spring, teliospores germinate and produce basidiospores, capable of infecting the alternate host of the genus Berberis spp., primarily common barberry (B. vulgaris), as well as few species of Mahonia (Mahonia spp.) (Martens, 1985). On the underside of barberry leaves aecia develop, where dikaryotic aeciospores are formed and attack graminaceous hosts. The grass host is continuously re-infected with single-celled dikaryotic urediniospores. At this stage, the rust reproduces asexually in repeated infection and sporulation cycles until plant maturity.
Environmental conditions prevailing in the geographical region and the presence or absence of the alternate host influences the diverse disease epidemiology in different parts of the world. In the absence of barberry but with year-round access of spores to the green grass host, clonal reproduction will dominate. When sexual reproduction is not possible, virulent new pathotypes can arise through a series of mutations (Figueroa et al., 2020). Clonal populations are found in Australia (Haque et al., 2008). In such conditions, the fungus population exposed to a strong selection may be dominated by several of the strongest genotypes (Pariaud et al., 2009).
Oat stem rust occurs worldwide and understanding the dynamics of the pathogen’s diversity is crucial to developing methods for its control. One way to prevent disease development is the use of fungicides. Another commonly used method to control the pathogen is alternate host eradication (Peterson et al., 2005). Plant breeding directed at genetic resistance seems to be a promising, environmentally friendly alternative. However, the development of resistance breeding strategies requires systematic virulence studies and detection of new pathotypes that break known resistance genes. A pathotype virulence profile is determined by inoculating a set of differentials, each having one single known resistance gene. Such tests conducted under laboratory conditions by the detached leaf method developed by Hsam et al. (1997) has been successfully used for virulence structure studies of oat diseases in Europe such as powdery mildew (Okoń & Ociepa, 2017) and crown rust (Paczos-Grzȩda & Sowa, 2019; Sowa et al., 2021; Sowa & Paczos-Grzęda, 2017). The last P. graminis virulence research in this region had been carried out in the years 1988 to 1996 (Šebesta et al., 1998), therefore the aim of this study was the update of pathogen’s level and structure of virulence as well as a preliminary evaluation of Pg race-specific resistance genes and their potential for use in practical breeding in the central and eastern European growing conditions.
Materials and methods
Experimental material
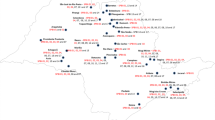
Samples of P. graminis f. sp. avenae were collected from infected oat stems in the years 2017–2020 from farm fields of three oat breeding companies in Poland (Polanowice 52° N, 16°81 E; Kopaszewo 50°2 N, 20°08 E; Strzelce 52°19 N, 19°25 E) and the experimental farm of the University of Life Sciences in Lublin (Czesławice 51°18 N, 22°15 E) (in red on the map in Fig. 1) as well as experimental stations of the Research Centre for Cultivar Testing (in black on the map in Fig. 1).
Each year up to three single-spore isolates were established under laboratory conditions from each collection site according to the host-pathogen method by Hsam et al. (1997), modified by Paczos-Grzȩda and Sowa (2019). Isolates were multiplied on ten-day old 3 cm long fragments of ‘Kasztan’ leaves placed on Petri dishes containing agar (0.6%) and benzimidazole (3.4 mM). Leaves were inoculated in a settling tower by spreading spores of P. graminis onto the plant material. Dishes were then incubated in a phytotron at 18 °C with 70% humidity and light intensity of approximately 4 kLx for a 16-h photoperiod.
Virulence assessment
Each single-spore isolate was examined on a set of 12 reference oat lines with a single stem rust resistance gene (Pg1, Pg2, Pg3, Pg4; Pg6, Pg8, Pg9, Pg10; Pg12, Pg13, Pg15, Pg16) (Fetch & Jin, 2007). Additionally, oat lines ‘Pg-a’, ‘Alpha’ (CI 9221), ‘Omega’ (CI 9139) (Martens et al., 1981) and ‘Wisconsin X-1588-2’ (CI 8457) were included in the differential set. Seeds were grown in plug trays filled with a universal substrate containing peat. Laboratory host-pathogen tests were carried out on the first leaves of 10-day-old seedlings of oat lines. Five leaf fragments, each from a different seedling of one oat line were placed onto 12-well culture plates filled with agar, with variety ‘Kasztan’ as infection control in each well. Inoculations were performed as described above.
After 12–14 days of incubation, stem rust disease symptoms were evaluated according to the scale of Stakman et al. (Stakman et al., 1962). Seedlings displaying infection types of 0;, 1, and 2 and their modifications (;1′, 11+, T... etc.) were classified as resistant, while those with ITs of 3 or 4 were considered susceptible. If a line revealed both resistant and susceptible ITs (mixed reaction) single-pustule isolates of each IT were generated to determine the identity of each race.
Data analysis
Population virulence was determined based on a number of compatible (virulent) reactions for the isolate on the set of differentials, as well as the number of unique virulence patterns describing different pathotypes. Virulence complexity was calculated as a number of Pg reference lines that have been infected by a number of isolates. Virulence frequency for each isolate was calculated as a percentage of all Pg genes overcame by this isolate. Frequency of virulence to each differential was defined as the percentage of isolates that infected this differential (Kosman, 1996, 2003). Population phenotypic diversity was assessed by Simpson Diversity Index (S) (Simpson, 1949), Evenness Index (EH) (Sheldon, 1969) and Kosman Index (Kosman, 1996, 2014) widely used for pathotype diversity analysis (Herrmann et al., 1999; Klocke et al., 2013; Manisterski et al., 2000; Paczos-Grzȩda & Sowa, 2019; Sheldon, 1969).
Each isolate was assigned to a three-letter code based on its reaction on the 12 differential lines (Table 1) (Fetch & Jin, 2007). Scores of infection were considered as virulence markers (Kosman & Leonard, 2007) and transformed into a binary 0/1 matrix (virulence = 1; avirulence = 0). Relationships between isolates as well as population diversity were visualized by principal coordinate analysis (PCoA) (Smith, 2002) based on estimators of standardized covariance of genetic distances (Dice, 1945) and 95% concentration ellipses were designated for each group. For hierarchical clustering, dissimilarity matrices were used to construct dendrograms using Ward’s method with 1000 bootstraps. The analysis was performed using Virulence Analysis Tool (VAT) software (Kosman et al., 2009; Schachtel et al., 2012), XLSTAT 2019.3.2 (Addinsoft, 2019) and PAST 3.19 software (Hammer et al., 2001).
Results
Stem rust did not occur every year in every region, and in consequence virulence comparison across all sites was not possible. There were 14 isolates collected in 2017, 30 in 2018, 22 in 2019 and 82 in 2020. Isolates were able to overcome from 5 to 11 differentials with the average virulence complexity of 8.6 (Fig. 2).
The frequency of virulence in 2017 ranged from 50% to 75% and on average was 66%. Isolates in 2018 were able to overcome differentials by 42% to 83% with an average value of 68%. In 2019 the frequency of virulence ranged from 50% to 83% with an average of 69%, while in 2020 it ranged from 42% to 92% and averaged 75%. According to the Kruskal–Wallis rank sum test, frequency of virulence in 2020 was significantly higher than that observed in 2017 and 2018 (p < 0.001). (Fig. 3).
Within 148 tested isolates, none were avirulent towards the full set of differentials used in the study. Fifty-seven pathotypes were identified, of which 36 were unique, meaning that the profile occurred only once in the tested set. A P. graminis race virulent to 11 oat differentials (TTK) was identified ten times and collected in 2020 in nine separate locations. TTK isolates were virulent to ‘Wisconsin X-1588-2’ and avirulent to Pg12, ‘Pg-a’, ‘Alpha’ and ‘Omega’. Thirty isolates were virulent to ten Pg genes, eleven of which wereavirulent to Pg6 and Pg12 (TKK) and fourteen were avirulent to Pg10 and Pg12 (TSK). The remaining five isolates were avirulent to Pg12 and displayed avirulence to Pg4, Pg9, or Pg16. Thirty-four out of 40 of the most virulent isolates were collected in 2020 in various locations in Poland. Forty-seven isolates were able to overcome nine Pg genes. Eight of these isolates were unique pathotypes with race SSK identified 19 times, race TJK identified 16 times, and races RSK and TSJ occurred twice each. The Simpson Index (S), indicating the number of different pathotypes (richness) and the degree of prevalence of some pathotypes (evenness) was 0.945. A similar value (0.85) was calculated for Evenness Index (EH), showing the degree of prevalence of some races observed among isolates. The Kosman Index (K) based on frequencies of individual virulences equalled 0.312 (Table 2).
The first two components of Principal Coordinate Analysis (PCoA) explained 34% of the variation. Samples were divided according to the year of collection. All four groups overlapped considerably, however, the group of samples from 2020 was distinct from the rest of the population (Fig. 4).
A similar pattern was observed in the Ward’s dendrogram (Fig. 5). This analysis grouped samples into two clearly separated clusters. The first is composed of 66 P. graminis isolates of which 59 were collected in 2020. Second cluster encompasses remaining samples.
In the years 2017–2019, the frequency of virulence to Pg resistance genes was relatively stable. An increase of virulence to Pg2, Pg4 and Pg10 was recorded in 2020. There was no isolate virulent to Pg12, ‘Pg-a’, ‘Alpha’ or ‘Omega’. Gene Pg10 was relatively effective, overcome by 33 P. graminis isolates collected in 2020, and had the same virulence profile as ‘Wisconsin X-1588-2’. A very low level of effectiveness was presented by genes Pg1, Pg2, Pg3, Pg6, Pg8, Pg9, Pg13, Pg15 and Pg16, with Pg1 and Pg3 being the most susceptible of all differential lines, overcome by 141 out of 148 isolates tested (Fig. 6).
Discussion
This is the first study of P. graminis f. sp. avenae virulence in a European country in 25 years (Šebesta et al., 1998), assessed on 148 isolates collected throughout Poland during the years 2017 to 2020. Šebesta and coworkers in their research of stem rust incidence in Europe from 1988 to 1996 tested virulence of four samples collected in Wielopole, Poland, where the disease was present in 1988, 1990 and 1996. Each sample was tested on 14 differential lines (Pg1, Pg2, Pg3, Pg4, Pg8, Pg9, Pg12, Pg13, Pg15, Pg16, ‘Pg-a’, A.strigosa cv. Saia, A. sterilis accessions VIR 343–1 (V1) and VIR 343–2 (V2)) and represented a different virulence combination. The first P. graminis sample was virulent to Pg3 and Pg9, the second sample was virulent to Pg1, Pg2, Pg3, Pg4, Pg8, Pg9, Pg12 and V1, the third sample overcame resistance genes Pg1, Pg2, Pg3 and Pg9 and the fourth sample was virulent to Pg1, Pg2, Pg3, Pg12 and Pg16. (Šebesta et al., 1998). In our study each isolate was examined on a set of 12 single-gene differentials (Fetch & Jin, 2007) as well as oat lines ‘Pg-a’, ‘Alpha’, ‘Omega’ (Martens et al., 1981) and ‘Wisconsin X-1588-2’ (Harder, 1999). Effectiveness of Pg10, ‘Alpha’, and ‘Omega’ has not been tested in a European country so far. The study showed a similar level of virulence in 2017–2019, on average around 68% (eight avr alleles), and the increase in virulence in 2020 to 75% (nine avr alleles). The increase in virulence over the years is seen with many rust pathogens, mainly as a consequence of the use of long-term monoculture cultivation practices with cultivars carrying single resistance genes (Van Niekerk et al., 2001; Park, 2008). A sudden increase in the distribution of a resistance gene over a wide geographical area (the ‘boom’) can lead to an epidemic caused by pathogen adaptation (the ‘bust’) in the so-called ‘boom and bust’ cycle (McDonald & Linde, 2002). However, oat cultivars bred in Poland are mostly susceptible to stem rust infection, thus the appearance of more virulent races is most likely not due to the selection pressure resulting from the prevalence of cultivars containing resistance genes.
A large proportion of the collected and tested material came from 2020. This was associated with a higher incidence and prevalence of the pathogen that year. In the growing season of 2020, the weather conditions (temperature and humidity) significantly favored the development of stem rust. The larger research sample size could more reliably reflect the range of the pathogen virulence.
Within 148 tested isolates, 57 races were identified, of which 36 occurred only once. Diversity parameters indicate differentiation of pathotypes, however similarities exist in the virulence profile between them. During the study, the most dominant race was SSK, virulent to nine Pg genes and avirulent to Pg4, Pg10 and Pg12. This race occurred every year of the research. The second most commonly occurring race was TJK, avirulent to Pg6, Pg10 and Pg12. This race was identified in 2018, 2019 and 2020. Higher frequency was also recorded for TSK (avirulent to Pg10 and Pg12) found 14 times, TKK (avirulent to Pg6 and Pg12) found 11 times and TTK (avirulent to Pg12) found 10 times. These pathotypes were within 40 of the most virulent races and were collected in 2020.
Genetic variability of P. graminis may be due to the ubiquitous presence of an alternative stem rust host, common barberry on which sexual propagation of the pathogen takes place. In Poland B. vulgaris is very common and evenly distributed (Mirek et al., 2002). Studies show that in regions where the alternate host is essential for the rusts survival, the genetic diversity within the pathogen population is high (Ali et al., 2014; Berlin et al., 2012; Berlin & Rahmatov, 2015). Moreover, it has been proven that phenotypically and genetically different individuals can be produced from a single aecial cluster (Berlin et al., 2017; Wang et al., 2016). No data are available to assess the role of the aecial stage on the alternate host in creating virulence diversity in eastern Europe, so the main source of inoculum in this region may also be migrating spores. Puccinia urediniospores can travel very long distances, transmitted by wind (Kolmer, 2005). Long-distance dispersal (LDD) of plant pathogens is known to occur in various parts of the world, one such example is the so-called ‘Puccinia pathway’ in North America (Harder & Haber, 1992). Studies in Europe show spore movements to be formed in two main migration paths, the western and the eastern tract. The West European tract has its origin in Morocco and follows the Atlantic coast to Great Britain, the Netherlands, and Scandinavia. The eastern tract starts south to Greece, proceeds to Bulgaria, and splits into two branches. A small branch goes west to the middle and upper Danubian Plain. The main branch runs through Ukraine, Poland and ends up in Scandinavia (Nagarajan & Singh, 1990; Zadoks, 1967a, 1967b). The long-range dispersal of Puccinia spores suggests that the virulence profile seen in Poland is representative of much of eastern Europe and beyond.
An additional goal of the study was a preliminary evaluation of Pg genes and their potential for use in practical breeding in European growing conditions. In the years 2017–2019 the frequency of virulence to most of the analysed Pg genes was at a high, relatively stable level. An increase in virulence recorded in 2020 was related to genes Pg2, Pg4 and Pg10. The most susceptible of all reference lines were Pg1 and Pg3, overcome by 141 out of 148 isolates tested. A very low level of effectiveness was also presented by genes Pg2, Pg6, Pg8, Pg9, Pg13, Pg15 and Pg16. Šebesta et al. (1998) have already detected virulence to all these genes except of Pg16 in Poland in the 1990s. According to Meldrum and Oates (1997) Pg1, Pg2, Pg3, Pg4, Pg8 have been broken down in Australia in the 1970s. The Pg13 was an effective source of resistance until 1992, virulence for ‘Pg-a’ was detected in 1996. The emergence of new virulent pathotypes resulted in the overcoming of these genes (Adhikari et al., 1999). To date, virulence had been recorded for all known seedling stem rust resistance genes in Australia (Adhikari et al., 2000; Park, 2008).
Long-term screening of P. graminis virulence is also carried out in Canada, where according to the latest studies from 2015 to 2019, Pg6, Pg10 and Pg16 remain effective (Fetch et al., 2020). Gene Pg6 was also assessed as effective in the virulence study of samples gathered in China during 2012–2013 while Pg16 was ineffective and Pg10 mostly ineffective, although the study sample only included 26 P. graminis isolates (Li et al., 2015). On the contrary, Pg10 and Pg16, along with Pg3 were effective in the study of P. graminis virulence in 1997–1998 in South Africa (Van Niekerk et al., 2001) and remained effective when the study was repeated in 2017–2018 (Boshoff et al., 2019). In our study, Pg10 was defeated by 33 P. graminis isolates collected in 2020 and had the same virulence profile as oat line ‘Wisconsin X-1588-2’. This line, in addition to Pg10, most likely carries Pg3 and Pg4 (Harder, 1999; Harder et al., 1995). Interestingly in South Africa between 1997 and 1998, with Pg10 and Pg3 being fully effective, ‘Wisconsin X-1588-2’ was susceptible to three out of four identified pathotypes (Van Niekerk et al., 2001).
During our 4 years study, no isolates overcoming the resistance of Pg12, ‘Pg-a’, ‘Alpha’ or ‘Omega’ were found. In the study conducted around 25 years ago, ‘Pg-a’ was also fully effective, but two of four identified races overcame the resistance of Pg12 (Šebesta et al., 1998). Pg12 is a recessive gene identified in A. sativa cv. Kyto (CI 8250) (Martens et al., 1968). ‘Alpha’ and ‘Omega’ originated from transgressive segregation in a cross between A. sterilis line S-66AB667 (CI 8377) expressing APR (Adult Plant Resistance) and cv. Kyto (Rothman, 1986). ‘Omega’ was crossed with cv. Kyto and allelism tests confirmed that the resistance of both is conditioned by the same locus (Fetch & Jin, 2007). ‘Pg-a’ (RL 996) is a selection from Rodney*5/Exter//Omega and has an additional, uncharacterized resistance gene(s) derived from A. sterilis. This additional gene(s) proved to be very effective, when ‘Pg-a’, ‘Alpha’ and ‘Omega’ remained resistant to Canadian P. graminis races TGN and TJN virulent to Pg12, identified in 2006 (Fetch et al., 2011). Over the years in Canada, Australia, South Africa and China, virulence to Pg12 significantly increased (Boshoff et al., 2019; Fetch et al., 2020; Li et al., 2015; Park, 2008), so probably races from these regions do not affect the structure of the eastern European P. graminis population.
Continuous emergence of new P. graminis races provide significant challenges for oat rust resistance breeding. Current sources of resistance to stem rust rapidly lose their effectiveness, so the new ones are urgently needed. P. graminis single-spore isolates collected during the study may be used for screening of wild oat accessions in the search for effective resistance genes. Host-pathogen tests used in this research have proved its utility in investigation of potential sources of new genes in diploid, tetraploid, and hexaploid wild Avena species (Okoń et al., 2014, 2018; Okoń & Ociepa, 2018; Paczos-Grzęda et al., 2018, 2021). Virulent and diverse P. graminis races obtained may allow to postulate the presence of known or potentially novel resistance genes and support oat breeding programs.
References
Addinsoft. (2019). XLSTAT 2019.4.1 statistical and data analysis solution. New York, USA. https://www.xlstat.com.
Adhikari, K. N., McIntosh, R. A., & Oates, J. D. (1999). Inheritance of the stem rust resistance phenotype Pg-a in oats. Euphytica, 105(2), 143–154. https://doi.org/10.1023/A:1003433915257.
Adhikari, K. N., McIntosh, R. A., & Oates, J. D. (2000). Distribution and temperature sensitivities of genes for stem rust resistance in Australian oat cultivars and selected germplasm. Australian Journal of Agricultural Research, 51(1), 75–83. https://doi.org/10.1071/AR99039.
Ali, S., Gladieux, P., Rahman, H., Saqib, M. S., Fiaz, M., Ahmad, H., Leconte, M., Gautier, A., Justesen, A. F., Hovmøller, M. S., Enjalbert, J., & de Vallavieille-Pope, C. (2014). Inferring the contribution of sexual reproduction , migration and off-season survival to the temporal maintenance of microbial populations : a case study on the wheat fungal pathogen Puccinia striiformis f . sp. tritici. Molecular Ecology, 23(3), 603–617. https://doi.org/10.1111/mec.12629.
Berlin, A., & Rahmatov, M. (2015). Sexual reproduction contributes to genotypic variation in the population of Puccinia graminis in Tajikistan. European Journal of Plant Pathology, 141, 159–168. https://doi.org/10.1007/s10658-014-0534-2.
Berlin, A., Djurle, A., Samils, B., & Yuen, J. (2012). Genetic variation in Puccinia graminis collected from oats, rye, and barberry. Phytopathology, 102(10), 1006–1012. https://doi.org/10.1094/PHYTO-03-12-0041-R.
Berlin, A., Samils, B., & Andersson, B. (2017). Multiple genotypes within aecial clusters in Puccinia graminis and Puccinia coronata: Improved understanding of the biology of cereal rust fungi. Fungal Biology and Biotechnology, 4(1), 3. https://doi.org/10.1186/s40694-017-0032-3.
Boshoff, W. H. P., Visser, B., Terefe, T., & Pretorius, Z. A. (2019). Diversity in Puccinia graminis f. sp. avenae and its impact on oat cultivar response in South Africa. European Journal of Plant Pathology, 155(4), 1165–1177. https://doi.org/10.1007/s10658-019-01845-5.
Dice, L. R. (1945). Measures of the amount of ecologic association between species. Ecology, 26(3), 297–302. https://doi.org/10.2307/1932409.
Fetch, T. G., & Jin, Y. (2007). Letter code system of nomenclature for Puccinia graminis f. sp. avenae. Plant Disease, 91(6), 763–766. https://doi.org/10.1094/PDIS-91-6-0763.
Fetch, T. G., Fetch, J. M., & Xue, A. (2011). Races of Puccinia graminis on barley, oat and wheat in Canada in 2006. Canadian Journal of Plant Pathology, 33(1), 54–60. https://doi.org/10.1080/07060661.2011.536650.
Fetch, T. G., Mitchell Fetch, J., Zegeye, T., & Xue, A. (2020). Races of Puccinia graminis on barley, oat, and wheat in Canada from 2015 to 2019. Canadian Journal of Plant Pathology, 00(00), 1–9. https://doi.org/10.1080/07060661.2020.1829066.
Figueroa, M., Dodds, P. N., & Henningsen, E. C. (2020). Evolution of virulence in rust fungi — Multiple solutions to one problem. Current Opinion in Plant Biology, 56, 20–27. https://doi.org/10.1016/j.pbi.2020.02.007.
Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001). PAST: Paleontological Statistocs software package for education and data analysis. Palaeontologia Electronica, 4(4), 1–9.
Haque, S., Park, R. F., Keiper, F. J., Bariana, H. S., & Wellings, C. R. (2008). Pathogenic and molecular variation support the presence of genetically distinct clonal lineages in Australian populations of Puccinia graminis f. sp avenae. Mycological Research, 112(6), 663–673. https://doi.org/10.1016/j.mycres.2008.01.015.
Harder, D. E. (1999). Usefulness of gene Pg10 as a source of stem rust resistance in oat breeding. Phytopathology, 89(12), 1214–1217. https://doi.org/10.1094/PHYTO.1999.89.12.1214.
Harder, D. E., & Haber, S. (1992). Oat diseases and pathologic techniques. In H. G. Marshall & M. E. Sorrells (Eds.), Oat Science and Technology - Agronomy Monograph No. 33 (pp. 307–425). Madison, WI, USA: American Society of Agronomy, Crop Science Society of America. https://doi.org/10.2134/agronmonogr33.c12.
Harder, D. E., Chong, J., & Brown, P. D. (1995). Stem and crown rust resistance in the Wisconsin oat selection X1588-2. Crop Science, 35, 1011–1015. https://doi.org/10.2135/cropsci1995.0011183X003500040014x.
Herrmann, A., Löwer, C. F., & Schachtel, G. A. (1999). A new tool for entry and analysis of virulence data for plant pathogens. Plant Pathology, 48(2), 154–158. https://doi.org/10.1046/j.1365-3059.1999.00325.x.
Hsam, S. L. K., Peters, N., Paderina, E. V., Felsenstein, F., Oppitz, K., & Zeller, F. J. (1997). Genetic studies of powdery mildew resistance in common oat (Avena sativa L.) I. Cultivars and breeding lines grown in Western Europe and North America. Euphytica, 96, 421–427.
Klocke, B., Flath, K., & Miedaner, T. (2013). Virulence phenotypes in powdery mildew (Blumeria graminis) populations and resistance genes in triticale (x Triticosecale). European Journal of Plant Pathology, 137, 463–476. https://doi.org/10.1007/s10658-013-0257-9.
Kolmer, J. A. (2005). Tracking wheat rust on a continental scale. Current Opinion in Plant Biology, 8(4), 441–449. https://doi.org/10.1016/j.pbi.2005.05.001.
Kosman, E. (1996). Difference and diversity of plant pathogen populations: A new approach for measuring.
Kosman, E. (2003). Measure of multilocus correlation as a new parameter for study of plant pathogen populations. Phytopathology, 93(12), 1464–1470. https://doi.org/10.1094/PHYTO.2003.93.12.1464.
Kosman, E. (2014). Measuring diversity: From individuals to populations: Mini-review. European Journal of Plant Pathology, 138(3), 467–486. https://doi.org/10.1007/s10658-013-0323-3.
Kosman, E., & Leonard, K. J. (2007). Conceptual analysis of methods applied to assessment of diversity within and distance between populations with asexual or mixed mode of reproduction. New Phytologist, 174(3), 683–696. https://doi.org/10.1111/j.1469-8137.2007.02031.x.
Kosman, E., Dinoor, A., Herrmann, A., & Schachtel, G. A. (2009). Virulence analysis tool ( VAT ) user manual. Ttp://www.tau.ac.il/lifesci/departments/plant_s/members/kosman/VAT.html.
Leonard, K. J., & Szabo, L. J. (2005). Stem rust of small grains and grasses caused by Puccinia graminis. Molecular Plant Pathology, 6(2), 99–111. https://doi.org/10.1111/j.1364-3703.2005.00273.x.
Li, T., Cao, Y., Wu, X., Chen, S., Wang, H., Li, K., & Shen, L. (2015). First report on race and virulence characterization of Puccinia graminis f. sp. avenae and resistance of oat cultivars in China. European Journal of Plant Pathology, 142(1), 85–91. https://doi.org/10.1007/s10658-014-0591-6.
Manisterski, J., Eyal, Z., Benyehuda, P., & Kosman, E. (2000). Comparative analysis of indexes in the study of virulence diversity between and within populations of Puccinia recondita f. sp. tritici in Israel. Phytopathology, 90(6), 601–607.
Martens, J. W. (1985). Stem rust of oats. In Alan P Roelfs & W. R. Bushnell (Eds.), The cereal rusts volume II diseases, distribution, epidemiology, and control (pp. 103–129). Academic.
Martens, J. W., McKenzie, R. I. H., & Fleischmann, G. (1968). The inheritance of resistance to stem and crown rust in Kyto oats. Canadian Journal of Genetics and Cytology, 10(4), 808–812. https://doi.org/10.1139/g68-101.
Martens, J. W., Rothman, P. G., McKenzie, R. I. H., & Brown, P. D. (1981). Evidence for complementary gene action conferring resistance to Puccinia graminis avenae in Avena sativa. Canadian Journal of Genetics and Cytology, 23(4), 591–595. https://doi.org/10.1139/g81-065.
McDonald, B. A., & Linde, C. (2002). The population genetics of plant pathogens and breeding strategies for durable resistance. Euphytica, 124, 163–180.
Meldrum, S., & Oates, J. D. (1997). Cereal rust survey 1997–1998, Annual Report.
Mirek, Z., Piękoś-Mirkowa, H., Zając, A., & Zając, M. (2002). Flowering plants and pteridophytes of Poland. In Z. Mirek (Ed.), Biodiversity of Poland (pp. 1–442). Szafer Institute of Botany PAS.
Nagarajan, S., & Singh, D. V. (1990). Long-distance dispersion of rust pathogens. Annual Review of Phytopathology, 28, 139–153. https://doi.org/10.1146/annurev.py.28.090190.001035.
Okoń, S. M., & Ociepa, T. (2017). Virulence structure of the Blumeria graminis f. sp. avenae populations occurring in Poland across 2010-2013. European Journal of Plant Pathology, 1–8., 149, 711–718. https://doi.org/10.1007/s10658-017-1220-y.
Okoń, S. M., & Ociepa, T. (2018). Effectiveness of new sources of resistance against oat powdery mildew identified in A. sterilis. Journal of Plant Diseases and Protection, 125(5), 505–510. https://doi.org/10.1007/s41348-018-0171-7.
Okoń, S. M., Chrząstek, M., Kowalczyk, K., & Koroluk, A. (2014). Identification of new sources of resistance to powdery mildew in oat. European Journal of Plant Pathology, 139(1), 9–12. https://doi.org/10.1007/s10658-013-0367-4.
Okoń, S. M., Ociepa, T., Paczos-Grzęda, E., & Ladizinsky, G. (2018). Evaluation of resistance to Blumeria graminis (DC.) f. sp. avenae, in Avena murphyi and A. magna genotypes. Crop Protection, 106(January), 177–181. https://doi.org/10.1016/j.cropro.2017.12.025.
Paczos-Grzȩda, E., & Sowa, S. (2019). Virulence structure and diversity of Puccinia coronata f. sp. avenae P. syd. & syd. in Poland during 2013 to 2015. Plant Disease, 103(7), 1559–1564. https://doi.org/10.1094/PDIS-10-18-1820-RE.
Paczos-Grzęda, E., Sowa, S., Koroluk, A., & Langdon, T. (2018). Characteristics of resistance to Puccinia coronata f. sp. avenae in Avena fatua. Plant Disease, 102(12), 1–9. https://doi.org/10.1094/pdis-03-18-0528-re.
Paczos-Grzęda, E., Boczkowska, M., Sowa, S., Koroluk, A., & Toporowska, J. (2021). Hidden diversity of crown rust resistance within Genebank resources of Avena sterilis L. Agronomy, 11(315), 1–14. https://doi.org/10.3390/agronomy11020315.
Pariaud, B., Ravigné, V., Halkett, F., Goyeau, H., Carlier, J., & Lannou, C. (2009). Aggressiveness and its role in the adaptation of plant pathogens. Plant Pathology, 58(3), 409–424. https://doi.org/10.1111/j.1365-3059.2009.02039.x.
Park, R. F. (2008). Breeding cereals for rust resistance in Australia. Plant Pathology, 57(4), 591–602. https://doi.org/10.1111/j.1365-3059.2008.01836.x.
Peterson, P. D., Leonard, K. J., Roelfs, A. P., & Sutton, T. B. (2005). Effect of barberry eradication on changes in populations of Puccinia graminis in Minnesota. Plant Disease, 89(9), 935–940. https://doi.org/10.1094/PD-89-0935.
Roelfs, A. P., & Martens, J. W. (1988). An international system of nomenclature for Puccinia graminis f. sp. tritici. Phytopathology, 78, 526–533.
Roelfs, A. P., Singh, R. P., & Saari, E. E. (1992). Rust diseases of wheat: Concepts and methods of disease management. Rust diseases of wheat: Concepts and methods of disease management. CIMMYT.
Rothman, P. G. (1986). Adequate rust resistance in oats. In Proceedings of the Second International Oats Conference (pp. 72–76). Springer Netherlands. https://doi.org/10.1007/978-94-009-4408-4_15.
Schachtel, G. A., Dinoor, A., Herrmann, A., & Kosman, E. (2012). Comprehensive evaluation of virulence and resistance data: A new analysis tool. Plant Disease, 96, 1060–1063.
Šebesta, J., Zwatz, B., Roderick, H. W., Harder, D. E., Corazza, L., & Stojanovic, S. (1998). Incidence of oat stem rust and virulence of Puccinia graminis pers. f. sp. avenae Erikss. et Henn. on oat and the effectiveness of resistances in Europe during 1988 to 1996. Archives of Phytopathology and Plant Protection, 31(5), 393–413. https://doi.org/10.1080/03235409809383251.
Sheldon, A. L. (1969). Equitability indices: Dependence on the species count. Ecology, 50(3), 466–467. https://doi.org/10.2307/1933900.
Simpson, E. H. (1949). Measurement of diversity. Nature, 163(4148), 688–688. https://doi.org/10.1038/163688a0.
Smith, L. (2002). A tutorial on principal components analysis. http://www.cs.otago.ac.nz/cosc453/student_ tutorials/principal_components.pdf.
Sowa, S., & Paczos-Grzęda, E. (2017). Puccinia coronata f. sp. avenae virulence in South-Eastern Poland in 2014. Folia Pomer. Univ. Technol. Stetin., Agric., Aliment., Pisc., Zootech., 336:43(3), 157–166. https://doi.org/10.21005/oe.2017.86.1.06.
Sowa, S., Paczos-Grzeda, E., & Paczos-Grzȩda, E. (2021). Virulence structure of Puccinia coronata f. sp. avenae and effectiveness of Pc resistance genes in Poland during 2017-2019. Phytopathology, Online ahe. https://doi.org/10.1094/PHYTO-10-20-0457-R.
Stakman, E. C., Stewart, D. M., & Loegering, W. Q. (1962). Identification of physiologic races of Puccinia graminis var. tritici. St. Paul (MN): USDA-ARS Misc. Publ. E-617 (revised).
Van Niekerk, B. D., Pretorius, Z. A., & Boshoff, W. H. P. (2001). Pathogenic variability of Puccinia coronata f. sp. avenae and P. graminis f. sp. avenae on oat in South Africa. Plant Disease, 85(10), 1085–1090. https://doi.org/10.1094/PDIS.2001.85.10.1085.
Wang, Z. Y., Zhao, J., Chen, X. M., Peng, Y. L., Ji, J. J., Zhao, S. L., et al. (2016). Virulence variations of Puccinia striiformis f. sp. tritici isolates collected from berberis spp. in China. Plant Disease, 100(1), 131–138. https://doi.org/10.1094/PDIS-12-14-1296-RE.
Zadoks, J. C. (1967a). Epidemiology of wheat rust in europe. Pest Articles and News Summaries, Section B: Plant Disease Control, 13(1), 29–46. https://doi.org/10.1080/05331846709432232.
Zadoks, J. C. (1967b). International dispersal of fungi. Netherlands Journal of Plant Pathology, 73(1 Supplement), 61–80. https://doi.org/10.1007/BF01974423.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This manuscript did not involve any human participants, and/ or animals.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sowa, S., Toporowska, J., Koroluk, A. et al. First detailed report on Puccinia graminis f. sp. avenae virulence structure and Pg resistance genes effective in Poland. Eur J Plant Pathol 161, 371–381 (2021). https://doi.org/10.1007/s10658-021-02329-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-021-02329-1