Abstract
Despite its major role in global isoprene emission, information on the environmental control of isoprene emission from tropical trees has remained scarce. Thus, in this study, we examined the relationship between parameters of G-93 isoprene emission formula (CT1, CT2, and α), growth temperature and light intensity, photosynthesis (ɸ, Pmax), isoprene synthase (IspS) level, and 2-C-methyl-d-erythritol 4-phosphate (MEP) pathway metabolites using sunlit and shaded leaves of four tropical trees. The results showed that the temperature dependence of isoprene emission from shaded leaves did not differ significantly from sunlit leaves. In contrast, there was a lower saturation irradiance in shaded leaves than in sunlit leaves, the same as temperate plants. The photosynthesis rate of shaded leaves showed lower saturation irradiance, similar to the light dependence of isoprene emission. In most cases, the concentration of MEP pathway metabolites was of lower tendency in shaded leaves versus in sunlit leaves, whereas no significant difference was noted in IspS level between sunlit and shaded leaves. Correlation analysis between these parameters found that CT1 of the G-93 parameter was positively correlated with the concentration of DXP and DMADP, whereas CT2 correlated with the concentration of MEP and the average air temperature for the past 48 h. Similarly, α closely associated with the initial slope (ɸ) of photosynthesis rate, and the basal emission factor is also linked to the photon flux of past days. These results suggest that growth conditions may control the temperature dependence of isoprene emission from tropical trees via the changes in the profiles of MEP pathway metabolites, causing alteration in the parameters of the isoprene emission formula.
Similar content being viewed by others
Introduction
Isoprene is the most abundant volatile organic compound emitted from terrestrial vegetation into the atmosphere (Guenther et al. 1995, 1996; Loreto and Fineschi 2015; Sharkey and Monson 2014); it has been identified to have severe impacts on tropospheric chemistry and ozone generation in both rural (Li et al. 2018; Reich and Amundson 1985; Runeckles and Chevone 1992) and urban landscapes (Fehsenfeld et al. 1992; Squire et al. 2015; Trainer et al. 1987). Additionally, isoprene is known to readily react with hydroxy radicals and other oxidizing species to indirectly extend the lifespan of methane, which is a greenhouse gas (Poisson et al. 2000). Tropical and subtropical regions account for half of the global annual isoprene input into the atmosphere due to year-round high temperatures and extreme light intensity (Guenther et al. 1995). However, despite this significant and primary role of tropical trees, studies on isoprene emission and its regulation have been mostly conducted on temperate tree species.
Though several factors are known to control isoprene emissions, the most important environmental factors that drive isoprene emission over a short-term period are temperature and light intensity (Monson and Fall 1989; Monson et al. 1992). The G-93 emission algorithm has been most extensively applied to estimate leaf-level isoprene emissions from mid-latitude forests (Benjamin et al. 1996; Geron et al. 2000; He et al. 2000; Xiaoshan et al. 2000) and tropical forests (Chang et al. 2005; Padhy and Varshney 2005; Rinne et al. 2002). There have been notions of whether one size fits all; i.e., whether these coefficients of G-93 (CT1 = 95,000, CT2 = 230,000, and α = 0.0027) fit all isoprene emission behaviors of both temperate and tropical plants (Arneth et al. 2008). Several studies have already reported differences between emission responses of tropical and temperate plants, bringing into question the efficacy of the G-93 formula and its application to tropical forests (Alves et al. 2014; Galan et al. 2009; Gupta et al. 2011; Lerdau and Keller 1997). The iterative optimization procedure successfully optimized the parameters of G-93 to sufficiently capture the temperature and light dependence of isoprene emission from the leaves of six tropical cut branches in our previous study (Higa et al. 2018). This procedure enables the authors to optimize the parameters of G-93 and characterize the isoprene emission behavior.
Isoprene is synthesized from the substrate dimethylallyl diphosphate (DMADP) supplied by the plastidial 2-C-methyl-d-erythritol 4-phopsphate (MEP) pathway using newly fixed carbon and reducing power supplied by photosynthesis. Therefore, the isoprene emission rate is considered to be a function of light intensities, temperature, and activities of photosynthesis, MEP pathway, and IspS. Controlling for isoprene emission is complex; however, it can be explained based on several regulatory levels: (1) isoprene synthase kinetics and enzyme activation: (2) gene expression of isoprene synthase and its protein level: and (3) substrate DMDP availability depending on the activity of the MEP pathway. Environmental control of isoprene emission is, therefore, a reflection and integration of these complex regulatory mechanisms. To better understand the environmental control of isoprene emission from tropical trees, it is important to examine the relationships between G-93 parameters and these factors under different growth conditions.
There have been several studies examining the control of isoprene emission from sunlit leaves because of their substantial contribution to the total emitted isoprene in response to changes in light intensity and temperature (Alves et al. 2014; Geron et al. 2002; Higa et al. 2018; Meeningen et al. 2016; Oku et al. 2015; Parveen et al. 2019a, b). In contrast to sunlit leaves, to date, only a few studies have focused on isoprene emission from shaded leaves. For example, Harley et al. (1997) reported no difference in the temperature responses of sunlit versus shaded leaves of Quercus alba, but with greater quantum efficiency and saturation at lower light intensity than sunlit leaves. The same group also compared the temperature response of sunlit leaves versus shaded leaves in sweetgum (Liquidambar styraciflua), wherein a higher amplitude of temperature response was noted for sunlit leaves versus shaded leaves (Harley et al. 1996). However, there was no quantitative analysis of the relationship between emission formula and growth condition parameters, photosynthesis, MEP pathway metabolite, and IspS protein level.
Understanding the environmental control of isoprene emission at the leaf-level is a prerequisite to improve the estimation of regional isoprene emissions. Furthermore, the shaded leaves account for a proportion of foliage of tropical trees and are a potential source of isoprene emission from tropical forests. However, there is yet no study attempting to parameterize the emission model of isoprene emission from shaded leaves of tropical trees. Here, we parameterized the G-93 emission formula for sunlit and shaded leaves of four tropical trees using an iterative optimization procedure. Furthermore, we examined their correlation with the parameters of growth condition and the MEP pathway metabolite to explore the environmental control of the isoprene emission formula for the first time.
Materials and methods
Plant materials
Cut branches were prepared from evergreen trees growing on Ryukyu University (26_15002.300N 127_45052.000E): Syzygium cumini (L.) Skeels, Syzygium samarangense (Blume) Merr. & L.M.Perry, Bauhinia variegata (L.) Benth., and Mangifera indica L.. Tree height was 5.0, 5.7, 7.5, and 4.3 m for S. cumini, S. samarangense, B. variegata, and M. indica, respectively. Ryukyu University is located in the subtropics with June to the beginning of October within a single summer and no wet and dry seasons. Tree species were the same as in our previous study (Higa et al. 2018). Sunlit leaves on the top of the tree and shaded leaves 2–3 m from the top were used for measurements. Cut branches were about 50 cm in length and were kept in tap water immediately after preparation. Measurements were made between September 2 and September 20 in 2018 for S. cumini; July 15 and July 24 in 2019 for S. samarangense; August 27 and August 30 in 2019 for B. variegata; and September 28 and October 3 in 2019 for M. indica. The leaves used were the 4th to 6th from the apex with a healthy, fully expanded, and distinctively mature appearance. Emission measurements were repeated three times using three cut branches of a tree for each species. Cut branch has been used for isoprene emission measurement in our previous study (Higa et al. 2018) and in many other studies (Affek and Yakir 2002; Geron et al. 2002; Kim et al. 2005; Monson et al. 1994). Measurement was completed in 3 h after branch cutting, and the emission was stable under given conditions of the light, temperature, and time regimes. As per the photosynthesis measurement using the cut branch, no abnormality was found in the gas exchange physiology of both sunlit and shaded leaves. Leaves growing in close vicinity to the branch used for isoprene emission measurements were also sampled to analyze the 2-C-methyl-d-erythritol 4-phosphate (MEP) pathway metabolites and photosynthetic activity. The leaves used for isoprene measurement were not used for metabolite analysis to avoid an artifact effect of the isoprene measurement involving two rounds of up and down light irradiance under laboratory conditions.
Light and temperature measurements of sunlit and shaded environments
The growth conditions of sunlit and shaded leaves were measured using a HOBOR Pendant Temperature/Light Data Logger (Onset Computer Corporation, MA, USA) as described previously (Oku et al. 2014). The logger was placed close to sunlit and shaded leaves to approximate light intensity, and data were logged at 10 min intervals for the past 10 days, including the isoprene measurement day. The illumination records on the logger were converted into photosynthetically active radiation (PAR) by the standard curve constructed based on the relationship between illumination intensity and PAR under comparable circumstances (correlation coefficient between illumination and PAR was 0.945, p < 0.001, Fig. S1).
Isoprene emission
Isoprene emission from an individual leaf of four tropical trees was analyzed using an online isoprene analyzer (KFCL-500, Anatec Yanako, Kyoto, Japan) as previously described (Higa et al. 2018). The analyzer was then calibrated with standard isoprene gas (17.52 ppm) purchased from Tokyo Koatsu Co., Tokyo, Japan, as recommended by the supplier: the first calibration at high-range mode using 17.52 ppm isoprene standard, followed by manual calibration of the low-range mode (200 ppb max). The calibration by this procedure usually gives a minimum detection sensitivity (2 δ) of 1.2 ppb for low-range measurements. Leaves were held by a leaf chamber (PLC-4C, Shimadzu, Kyoto, Japan), and measurements of isoprene emission, leaf temperature, and light intensity were monitored simultaneously as previously described (Higa et al. 2018). Light intensity was measured using a quantum light sensor (Spectrum Technologies Inc., IL, USA) on the leaf chamber, and leaf temperature was measured by thermocouples (type T). Ambient fresh air was pumped into the leaf chamber at a flow rate of 400 mL/min by a mini-pump (MP-Σ30, Shibata Scientific Technology Ltd., Saitama, Japan), and the outlet flow from the chamber was introduced through a three-way valve into the isoprene analyzer with a flow rate of 100 mL/min. Isoprene in the outlet flow reacted with ozone to produce chemiluminescence, and the luminescence intensity was monitored online via a blue-sensitive photomultiplier tube (Hills and Zimmerman 1990). Isoprene emission was analyzed using the low-range mode, and all measurements were corrected by subtracting the background emission level of isoprene (2–3 ppb) in ambient air. The changes in the background level during the measurement were usually less than 1 ppb and remained within the range of minimum detection sensitivity (2 δ = 1.2 ppb). It could be anticipated that the selectivity between isoprene and other alkenes would be insufficient. However, the selectivity of isoprene relative to other volatile compounds was found to be adequate in practice because of its rapid reaction and high efficiency to emit chemiluminescence (Hills and Zimmerman 1990).
Cut branches were placed in a phytotron (Koito Manufacturing Co. LTD, Tokyo, Japan), and the leaves were irradiated with LED light (CS Specialized Equipment Co. Sapporo, Japan) with 5 min stepwise variation in light intensity. The lighting program consisted of 25 steps and 2 cycles of up and down, as shown in Fig. S2. At each step, light intensity was constant for 5 min. Leaf temperature was allowed to vary, along with light intensity, as exemplified in Fig. S3. Isoprene emission and leaf temperature were recorded every 10 s, and light intensities acquired every 1 min were averaged for 5 min and used to optimize G-93 parameters as described previously (Mutanda et al. 2016a).
Parameterization of G-93
The model G-93 estimates isoprene emission (I) as
where I is the emission rate predicted at temperature T (K) and (PAR) of L (μmol m−2 s−1) and Is is the basal emission factor (emission factor) (Guenther et al. 1993). The two variables CT and CL refer to temperature and light coefficients, respectively, and are defined by
where CT1 = 95,000 J mol−1, CT2 = 230,000 J mol−1, Tm = 314 K, α = 0.0027, CL1 = 1.066, R = 8.314 J K−1 mol−1, and Ts are the leaf temperature at standard conditions (303 K).
The parameters of CT1, CT2, and α define the amplitude and shape of the temperature and light response curves of the G-93 emission model, and the constants listed above are empirical coefficients determined using nonlinear best fit methods using observations from temperate trees. Our previous study has successfully optimized the G-93 parameters for tropical trees using an iterative method named “Ping-Pong”, which consists of a mutual step-by-step optimization of the parameters listed above: CT1, CT2, and α. The robustness and accuracy of this method have been evaluated in our previous paper (Mutanda et al. 2016a). The authors here applied this procedure to parameterize the G-93 model and characterize the temperature and light response of both the sunlit and shaded leaves of four tropical trees. The convergence criterion of parameters was as described previously (Mutanda et al. 2016a). However, the parameter α for shaded leaves often increased more than 0.1; nevertheless, the actual change in CL was almost negligible. In these cases, the endpoint criterion of iteration for α was that the change in CL is less than 0.00001.
Photosynthesis
The leaves’ photosynthesis was measured using a CI-340 hand-held photosynthesis system (CID Bio-Science, Inc., WA, USA, imported by Ogawa Seiki Co. Tokyo, Japan). Leaves were first irradiated with light intensity of 500 μmol m–2 s–1 for 30 min; subsequently, the light intensity was varied stepwise from 0 to 1800 μmol m–2 s–1 with 7 min intervals: 0, 50, 100, 200, 300, 400, 500, 700, 900, 1200, 1500, and 1800 μmol m–2 s–1. The correlation between photosynthesis rate and light intensity was simulated by the rectangular hyperbola formula below (4)
where Pn is the photosynthesis rate at the light intensity of I. Parameters of ɸ, Pmax, and R represent the initial slope of the light response curve, maximal photosynthesis rate, and dark respiration rate, respectively, determined by the best fit nonlinear regression algorithm described elsewhere (Causton and Dale 1990). A non-rectangular formula has been reported to better fit the response curve of photosynthesis, showing saturation at low light intensity (Arntz et al. 2000). The authors, therefore, first tried the non-rectangular hyperbola formula to simulate the light-photosynthesis rate relationship of tropical trees. However, the nonlinear regression fit of the non-rectangular formula sometimes gave a negative value for the parameter of curvature (θ) especially with tree species that reached saturation at higher light intensity. The problem with the rectangular formula has been reported to be its higher estimation of Pmax (Arntz et al. 2000). However, the authors found largely comparable estimates of Pmax with both the rectangular and non-rectangular formulas for tropical tree species. Therefore, the rectangular formula was used to simulate the light response curve to compare the photosynthesis parameters for all tree species and sunlit and shaded leaves.
Chlorophyll fluorescence
Chlorophyll fluorescence was measured using a mini PM photosynthesis meter (Opto Science, Inc. Tokyo). The leaves sampled were covered with aluminum foil for 1 h before measuring the light response. The photosynthesis meter automatically generates 12 levels of irradiance, between 70 and 1700 μmol m–2 s–1, and measures the light-adapted Fm and F at a given light intensity. The quantum yield of photosynthesis (Φp) was calculated as (Causton and Dale 1990)
The photosynthesis rate (P) at the given light intensity (PAR) was estimated by the formula
2-C-methyl-d-erythritol 4-phosphate (MEP) pathway metabolite analysis
Polar intermediate metabolites of the MEP pathway were analyzed as described previously (Mutanda et al. 2016b). Leaf (300 mg) was ground in liquid nitrogen and homogenized with an extraction solvent containing acetonitrile/methanol/50 mM ammonium acetate (2:1:1) and pH 10 and centrifuged at 4 °C for 10 min at 11,600×g. The pH of the supernatant was adjusted to 6.8 by acetic acid and filtered through a 0.2 µm polyethersulfone membrane filter (GE Healthcare, UK). The filtrate was loaded onto the LC-NH2 solid-phase extraction cartridge (Sigma-Aldrich, Mo, USA) conditioned earlier by methanol and a double volume of Milli-Q at pH 6.8. The solid-phase cartridge was washed twice with Milli-Q (pH 6.8). Then, the metabolites were eluted with a mixture of methanol/Milli-Q (1:1 v/v) at pH 11.6 and dried using a vacuum speed evaporator at 4 °C. The metabolites were re-extracted in ethanol, dried up to 50 µL volume, mixed with an equal amount of acetonitrile, and then transferred to an injection vial. Ultrafast liquid chromatography-tandem mass spectrometry (UFLC-MS/MS) was used to quantify the polar metabolites of the MEP pathway using the method of Li and Sharkey (Parveen et al. 2019b) and as previously described (Mutanda et al. 2016b). The column used was a hydrophilic interaction liquid chromatography column (ZIC-pHILIC PEEK; 5 μm, 50 × 2.1 mm) (Merck SeQuant, Umeå, Sweden), with a HILIC guard column (5 μm, 20 × 2.1 mm). The solvents used were 50 mM ammonium acetate (pH 10) with LC–MS grade ammonium hydroxide as solvent A and acetonitrile as solvent B. Separation was achieved with a flow rate of 0.2 mL min–1 and column temperature of 25 °C. Identification and quantification of metabolites were achieved using authentic MEP pathway metabolite standards: 1-deoxy-d-xylulose 5-phosphate (DXP); MEP; 2-C-methyl-d-erythritol-2,4-cyclodiphosphate (MEcDP); 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate (HMBDP); and dimethylallyl diphosphate (DMADP) purchased from Echelon Biosciences (Logan, UT, USA).
IspS protein level
IspS protein level was estimated via Western blotting as described previously (Parveen et al. 2019b). Leaf tissue (40 mg) was homogenized in ice‐cold lysis buffer (50-mM Tris–HCl, pH 7.5; 5 mM ethylenediaminetetraacetic acid (EDTA), and 5-mM dithiothreitol), and the homogenate was centrifuged for 5 min. Proteins (15 μg) were separated on a 10% sodium dodecyl sulfate–polyacrylamide gel. The proteins were transferred to a polyvinylidene difluoride membrane, followed by blocking with 0.3% skim milk in Tris-buffered saline with Tween 20 (TBS-T) at room temperature for 1 h. Next, the membrane was incubated using an anti-F. septica IspS polyclonal primary antibody diluted 1:5000 in 0.3% skim milk. After washing five times in TBS-T buffer, the membranes were incubated for 1 h (room temperature) with a secondary antibody (anti-Guinea pig IgG, GeneTex, Irvine, CA, USA). ECL Advance Western Blotting Detection System (GE Healthcare) was used to detect the protein bands, and the image was visualized using an ImageQuant™ LAS4000 imaging system (GE Healthcare) with 120 s exposure time. Band intensity was quantified using ImageJ software (NIH, Bethesda, MD). The primary polyclonal antibody raised against F. septica IspS reacted with all IspS from four tree species. However, the titer may show variation between tree species. Thus, the comparison is considered valid in the same species, between sunlit and shaded leaves in this study.
Statistical analysis
Statistical significance of the differences between means was analyzed via non-parametric method (Mann–Whitney U test). Correlations between parameters were tested using the Pearson product-moment correlation coefficient, and Student’s t test checked the statistical significance. The criterion of statistical significance was p < 0.05 for both cases. Nonmetric multidimensional scaling (NMDS) was performed based on the distance of 1 − |r| using Excel 2010 (Higa et al. 2018).
Results
Growth conditions
Diurnal variation of light and air temperature of four tropical trees for the past 10 days of measurement of isoprene emission (including measurement day) is shown in Fig. 1. The light intensity peaked around midday, except for S. samarangense, and the highest irradiance of sunlit leaves for 10 days was 2205, 1402, 2022, and 1776 μmol m–2 s–1 for S. cumini, S. samarangense, B. variegata, and M. indica, respectively. The average irradiance of sunlit leaves during daytime (06:00–18:00) was 715, 334, 752, and 597 μmol m–2 s–1, while the average irradiance of shaded leaves was 220, 81, 138, and 97 μmol/m2/s for S. cumini, S. samarangense, B. variegata, and M. indica, respectively. Shade leaves’ irradiances were 20%–50% of sunlit leaves for past 10 days of the measurement (Fig. 1a1–d1).
Growth conditions of sunlit (black line) and shaded (gray line) leaves for the past 10 days of isoprene measurement (including the measurement day). a1–d1 and a2–d2 show diurnal changes in PAR (μmol m–2 s–1) and temperature (℃), respectively. Day 0 denotes the measurement day. Data are mean ± SE of three measurements
Air temperature for sunlit and shaded leaves was broadly comparable as shown in Fig. 1a2–d2, and the average air temperature at daytime (06:00–18:00) was 29.2 °C, 30.2 °C, 30.3 °C, and 28.4 °C for S. cumini, S. samarangense, B. variegata, and M. indica, respectively. Similarly, the highest air temperature during the daytime was 32.0 °C, 34.5 °C, 33.6 °C, and 31.4 °C and the lowest of 24.8 °C, 24.5 °C, 26.1 °C, and 23.4 °C for S. cumini, S. samarangense, B. variegata, and M. indica, respectively.
Parameterization of G-93
Temperature and light responses of sunlit and shaded leaves were examined using the leaves of cut branches as described previously (Higa et al. 2018). Leaves were irradiated for 5 min intervals using a lighting scheme, as illustrated in Fig. S2. The light intensities were varied from the lowest 200 to the highest 1200 or 1400 μmol m–2 s–1 in 25 steps and two rounds of up and down treatment. The leaf temperature varied from around 27 °C to 40 °C, depending on the ambient temperature and light intensity at a given time of the lighting scheme. The typical isoprene emission profile, PAR, and leaf temperature variation during the irradiation course are shown in Fig. S3.
The optimized parameters of G-93 via Ping-Pong method for sunlit and shaded leaves of the four tropical trees were listed in Table 1 and Fig. 2 show the normalized temperature and light responses of isoprene emissions predicted by G-93 with these optimized and default parameters. There was no statistically significant difference in the parameters of CT1 of sunlit versus shaded leaves with all tree species, whereas a difference in CT2 was noted between sunlit and shaded leaves of B. variegata (Table 2). Optimized α of the sunlit leaves was significantly lower than that of shaded leaves except for B. variegata. Notably, shaded leaves of S. cumini and S. samarangense tended to show lower temperature response compared to sunlit leaves, whereas the shaded leaves of M. indica showed a higher tendency in the temperature response than sunlit leaves (Fig. 2). The temperature response of sunlit and shaded leaves was almost comparable with B. variegata. The temperature dependence of both sunlit and shaded leaves of four trees showed a significantly higher response and substantial deviation from that predicted by G-93 of default parameters (Fig. 2).
Effect of temperature (a1–d1) and light intensity (a2–d2) on isoprene emission from sunlit (open circle) and shaded leaves (closed circle) of tropical trees. Panels a1–d1 and a2–d2, respectively, illustrate CT and CL functions, with mean parameters listed in Table 1. Dotted line denotes G-93
The light dependence of isoprene emission from shaded leaves showed saturation at lower light intensities due to a higher α value than sunlit leaves (Fig. 2). This trend was most clearly shown with the shaded leaves of B. variegata: isoprene mission of sunlit leaves saturated at higher light intensities. In contrast, shaded leaves saturated at lower light intensity than the default G-93 prediction (Fig. 2).
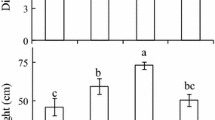
The basal emission factor (emission under conditions of 1000 mmol m–2 s–1 PAR and 30 °C) of tropical trees was predicted by optimized G-93 and illustrated in Fig. 3. The basal emission factors of shaded leaves were much lower than those of sunlit leaves: the lowest was 34% of sunlit leaves for S. samarangense, the medium was 41% for S. cumini, and the highest was 54% for S. variegata and M. indica (Fig. 3). The considerably lower basal emission factors, as compared to many other species, may be explained by overestimation of the basal emission rate by default G-93 for other species as discussed in our previous study (Mutanda et al. 2016a).
Basal emission factors of sunlit and shaded leaves of tropical trees. Data are predicted using the parameters listed in Table 1. Statistical significance was assessed by U test. *Significantly different from the sunlit leaf with p < 0.05
Photosynthesis rate
The bulk of the emitted isoprene is synthesized de novo from newly fixed carbon mobilized from photosynthesis through the methylerythritol phosphate (MEP) pathway that supplies dimethylallyl diphosphate (DMADP), the substrate of isoprene synthase. Thus, photosynthesis is an essential factor that controls the isoprene emission, and the photosynthesis rate of four tropical trees was measured (Fig. 4). The light dependence of the photosynthesis rate was simulated by a rectangular hyperbola formula (4), as mentioned in the materials and methods section. This formula resembles the characteristics of enzyme kinetics of Michaelis–Menten that draws an analogy between photosynthesis and enzyme reactions as a biochemical conversion of substrate or photon flux density (Guenther et al. 1993). The parameters for this formula are given in Table 2. Thus, half-saturation point equivalence to Km of the Michaelis–Menten formula was calculated to characterize the saturation kinetics. Initial slope (ɸ) of shaded leaves gave higher values than sunlit leaves in all species, indicating a steeper increase in photosynthesis rate with increasing photon flux at lower light intensity. In all species, the maximum photosynthesis rate (Pmax) of sunlit leaves showed higher values than shaded leaves. The half-saturation point of shaded leaves was significantly lower than that of sunlit leaves with S. cumini, S. samarangense, and B. variegata, but not with M. indica (Table 2). Similar trends of low light compensation points for shaded leaves were also noted with S. cumini, S. samarangense, and B. variegata (Table 2).
Effect of light intensity on photosynthesis rate of sunlit (open circle) and shaded (closed circle) leaves of tropical trees. Panels a–d1 illustrate the rectangular function of Pn (photosynthesis rate), with mean parameters listed in Table 2
The results of chlorophyll fluorescence measurements are summarized in Fig. S4. The quantum yield of both sunlit and shaded leaves was noted to rapidly decrease as the light intensity increased in all four tropical trees. However, the decay of the quantum yield in shaded leaves was steeper with increasing light intensity than sunlit leaves. The difference between sunlit and shaded leaves was less pronounced in S. cumini than in other tree species (Fig. S4). The photosynthesis rate based on the photosystem II quantum yield showed more clear-cut differences between sunlit and shaded leaves (please note the difference between the photosynthesis rate of Fig. 4 and of Fig. S4). Overall, the photosynthesis rate in sunlit leaves increased and saturated around 500 μmol m–2 s–1, and, after that, it decreased with increasing light intensity. In contrast, the photosynthesis of shaded leaves saturated around or below 200 μmol m–2 s–1 except for S. cumini where the saturation intensity was rather close to that of sunlit leaves (Fig. S4).
IspS protein level
The relative IspS protein levels in sunlit and shaded leaves of four tropical trees are shown in Fig. 5. No statistically significant difference was noted in the IspS protein level between sunlit and shaded leaves throughout the four tree species. However, the sunlit leaves of S. cumini, S. samarangense, and M. indica tended to show higher IspS levels compared to shaded leaves, whereas the sunlit leaves of B. variegata exhibited lower IspS levels compared to shaded leaves. The anti‐F. septica IspS polyclonal primary antibody was used to detect IspS of four tree species. Although this antibody reacted with all IspS from four species, the titer may show variation between species. Thus, the comparison is valid for the same species, between sunlit and shaded leaves. Because of this uncertainty, the parameter of IspS level was excluded as a parameter for correlation analysis.
MEP pathway metabolites
Metabolite pools of the MEP pathway (Fig. 6) were analyzed (for an overview of isoprene biosynthesis, refer to Lantz et al. 2019) to get more insights into the environmental control of isoprene emission. Among the metabolites, the concentration of DMADP and HMBDP in shaded leaves was significantly lower than that in sunlit leaves except for S. cumini (Fig. 7). The concentration of DMADP in shaded leaves of S. cumini was higher than that in sunlit leaves. No statistically significant difference was noted between sunlit and shaded leaves with other MEP pathway metabolites. The percentage of DMADP concentrations in the shaded versus sunlit leaves was 62.4% for S. samarangense and was about 25.0% for B. variegata and M. indica.
Schematic representation of isoprene biosynthesis. The abbreviations of the enzymes and metabolites involved in the MEP pathway are as follows: DXS (1-deoxy-d-xylulose-5-phosphate synthase), DXR (1-deoxy-d-xylulose-5-phosphate reductoisomerase), MCT (2-C-methyl-d-erythritol-4-phosphate cytidylyltransferase), CMK (4-cytidine-5′-diphospho-2-C-methyl-d-erythritol kinase), MCS (2-C-methyl-derythritol-2,4-cyclodiphosphate synthase), HDS (4-hydroxy-3-methylbut-2-enyl-diphosphate synthase), HDR (HMBDP reductase), IDI (isopentenyl diphosphate isomerase), and IspS (isoprene synthase). DXP (1-deoxy-d-xylulose-5-phosphate), MEP (2-C-methyl-d-erythritol 4-phosphate), CDPME (4-(cytidine 5′-diphospho)-2-C-methyl-d-erythritol), CDPMEP (2-phospho4-(cytidine 5′-di-phospho)-2-C-methyl-d-erythritol), MEcDP (2-C-methyl-d-erythritol 2, 4-cyclodiphosphate), HMBDP (4-hydroxy-3-methylbut-2-butenyl 4-diphosphate), DMADP (dimethylallyl pyrophosphate), and IDP (isopentenyl diphosphate)
Correlation analysis
The correlation among the parameters related to isoprene emissions was analyzed throughout the sunlit and shaded leaves of four tropical trees and summarized in Table 3 (data of Tables 1 and 2 and Figs. 1, 3, 7 were used for analysis). CT1 positively correlated with the concentration of DXP (p = 0.008) and DMADP (p = 0.059), whereas CT2 similarly correlated with the average air temperature for the past 48 h (p = 0.017) and MEP concentration (p = 0.000). Alpha (α) parameter of CL positively correlated with initial slope (ɸ) (p = 0.012) and negatively correlated with basal emission factor (p = 0.050) and the half-saturation point of photosynthesis rate (p = 0.034). The basal emission factor estimated using optimized G-93 positively correlated with Pmax (p = 0.010), half-saturation point (0.007), and growth irradiance of past10 days of emission measurements (p = 0.004). Notably, the concentration of DXP showed a positive correlation with DMADP, the substrate for isoprene biosynthesis (p = 0.049).
Nonmetric MDS
The relationship between these parameters was ordinated in two dimensions via nonmetric multidimensional scaling (NMDS) based on correlation coefficients listed in Table 3. The correlation matrix of 1 − |r| was the distance for NMDS input.
There seem to be three clusters related to CT1, CT2, α and basal emission factor (clusters 1, 2, and 3), as shown in Fig. 8. CT1 has been determined to be closely related to the concentration of DXP and DMADP, the first and last metabolites of the MEP pathway, in cluster 1, while CT2 is located closest to the concentration of MEP, which is the intermediate metabolite of the MEP pathway in cluster 2. The concentration of HMBDP and MEcDP is found between clusters 1 and 2. In contrast, α the coefficient of CL and basal emission factor (BEF), formed cluster 3 with photosynthesis parameters, ɸ, half-saturation point, maximum photosynthesis rate (Pmax), and the average growth irradiance of the past 48 and 240 h. Notably, the BEF formed a cluster with the parameters related to photosynthesis and light growth conditions, but not with the parameters of MEP pathway metabolites.
Two-dimensional plots of nonmetric multidimensional scaling (NMDS). The correlation coefficients listed in Table 3 (1 − |r|) were the input data for nonmetric MDS analysis. Open circle, factors related to photosynthesis and photon flux; open square, factors associated with temperature; triangle, MEP pathway metabolite; closed circles, G-93 parameters; T48, the average temperature for 2 days, including measurement day; T240, the average temperature for 10 days, including measurement day; L48, average PAR for 2 days, including measurement day; L240, average PAR for 10 days, including measurement day; BEF, basal emission factor; HS, half-saturation point; LC, light compensation point; R, dark respiration rate; Pmax, maximal photosynthesis rate. The solid line denotes a statistically significant correlation at p < 0.05, Dotted line denotes the correlation at p < 0.10
Because there is a strong correlation between CT2 and average air temperature in the past 48 h, we further examined this relationship for the past 10 days (Fig. 9a). As per our findings, the correlation coefficient decreased linearly with an increase in the number of past days. A statistically significant correlation with a criterion of p < 0.05 was only obtained up to the past 5 days (including the measurement day). Similarly, the correlation between the basal emission factor (BEF) and average irradiance of past days was studied (Fig. 9b). In contrast to the case for CT2, the correlation coefficient was only significant over more than the past 7 days and tended to increase with the number of past days up to 10 (including the measurement day).
Correlation coefficients as a function of the averaged past days. Averaged past day 1 denotes measurement day. For example, CT2 versus averaged air temperature (a), the basal emission factor (BEF) versus averaged PAR (b). Student’s t test evaluated the statistical significance of the correlation coefficient
Discussion
This study examined the temperature and light response of isoprene emission from sunlit and shaded leaves of four tropical trees growing on Okinawa Island, Japan. Harley et al. (1997) studied the temperature and light dependence of isoprene emission from sunlit and shaded leaves of oak and observed no difference in the temperature response of sunlit versus shaded leaves, whereas lower saturation irradiance in shaded leaves than in sunlit leaves was observed. This study found largely similar results on the temperature and light dependence of isoprene emission from tropical trees (Fig. 2). However, the parameters of CT1 and CT2 for tropical trees listed in Table 1 were much larger than the default values, as the authors observed in previous studies (Higa et al. 2018; Mutanda et al. 2016a).
CT1 of G93 originates from the equation to simulate the temperature response of enzymatic reactions (Guenther et al. 1993). Figure 10 depicts the typical changes in the shape of the normalized emission rate when only CT1 or CT2 was increased from the default value of 95,000 and 230,000 to 200,000 and 400,000, respectively. CT1 mainly defines the amplitude of the temperature dependence of isoprene emission, and the larger value indicates the higher amplitude of temperature response (Fig. 10a). CT2 relates to the shape of the response curve, and the curve slope becomes steep as the value increases (Fig. 10b). Although no statistical significance was noted for CT1 and CT2 between sunlit and shaded leaves of all tree species, a lower tendency in terms of temperature dependency was determined with the shaded leaves of S. cumini and S. samarangense, thus suggesting a higher amplitude of temperature response in isoprene emission (Fig. 2). Furthermore, CT1 showed a weak negative correlation with the BEF and a positive correlation with the concentration of DMADP (Table 3). This implies that DMADP concentration is not necessarily a hallmark for the BEF, as exemplified with the sunlit leaves of S. samarange. It is also worth noting that the concentration of substrate DMADP and MEP pathway intermediates may control the temperature response of isoprene emission. The reservoir of an intermediary, such as MEP, may be linked to the flux rate and regulate the instantaneous response of isoprene emission.
In contrast to CT1 and CT2, α parameter of CL showed statistically significant differences between sunlit and shaded leaves of S. cumini, S. samarangense, and M. indica, but not with V. variegata (Table 1). Alpha (α) defines the slope (steepness) of the light response curve, and the saturation irradiance decreases as α increases. This observation was also similar to the result of Harley et al. on the temperate plant oak. However, the saturation irradiance of shaded leaves for tropical trees in this study appears to be much lower than that of the temperate plant: below 200 μmol m−2 s−1 for tropical trees versus around 500 μmol m−2 s−1 temperate plants. The maximal growth irradiance for shaded leaves in this experiment was mostly below 200 μmol m–2 s–1, as shown in Fig. 1, and agrees well with the lower saturation irradiance. Thus, shaded leaves of tropical trees responded to temperature change to almost the same amplitude under low and small changes in growth irradiance.
To get more insights into the control mechanism of isoprene emission, the relationships between parameters obtained in this study were summarized in Table 3. They were visualized as a two-dimensional display (Fig. 8) by the nonmetric multidimensional scaling (NMDS) method. The correlation coefficients listed in Table 3 (1 − |r|) were the input data for MSD analysis. The distance between factors in Fig. 8 indicates inter-parameter relationships, and a closer distance between two parameters in the plot denotes a higher correlation.
The parameters have formed three distinct clusters in the plot. The MEP pathway metabolites DXP and DMADP were in the same cluster with CT1, suggesting that these factors are related to each other in terms of regulating isoprene emission. Because CT1 defines the amplitude of the temperature dependence of isoprene emission, it may be conceivable that the concentration of DXP and DMADP controls the amplitude of temperature dependence of isoprene emission in tropical trees. DXP is synthesized by DXP synthase (DXS), the main rate-limiting enzyme of the MEP pathway (Banerjee et al. 2013; Wright et al. 2014), whereas DMADP is the last metabolite in the pathway and is the substrate for isoprene synthase for isoprene biosynthesis in the chloroplast (Fig. 6). Thus, it appears that the amplitude of temperature dependence was controlled by the concentration of these metabolites in the chloroplast. However, it has been reported that the chloroplastic pool of DMADP is about one-third of the total pool, and whole leaf DMADP content cannot be used to predict the isoprene emission rate (Loreto et al. 2004). It seems to be an accepted view that there is limited crosstalk between the MEP pathway metabolites in the chloroplast and the cytosolic pool (Rodriguez-Concepcion and Boronat 2015). Notably, only DMADP and DXP derived from the MEP pathway showed a strong correlation with CT1 (r = 0.85, p = 0.007). Thus, crosstalk between chloroplasts and cytosol likely resulted in a rather redundant correlation between DMADP and isoprene emission (r = 0.68, p = 0.059). Despite the distinct chloroplastic and cytosolic pools of the intermediates, the total amount of DMADP could be a rough basis for predicting the temperature dependence of isoprene emission across plant species given that the partitioning between the chloroplast and cytosol is constant throughout the plant kingdom (e.g., 30% in chloroplasts versus 70% in the cytosol) (Bruggemann and Schnitzler 2002). This line of reasoning warrants further studies in the future.
Interestingly, CT2 formed another cluster with MEP and average air temperature of the past 48 and 240 h, suggesting that the concentration of these compounds and the past days’ air temperature are related to the peak slope of the temperature dependence curve. Predicted CT2 of tropical trees were higher than the default value of 230,000. The peak slope becomes steep with increases in CT2. The emission rate peaks in a narrow range of leaf temperatures, accompanied by slightly increased response amplitude and shift in the maximal temperature. Of the parameters in this cluster, MEP is the metabolite of DXP and showed the highest correlation with CT2 (r = 0.947, p = 0.000) (Table 3), suggesting that the MEP pathway flux is linked once more to the temperature response of isoprene emission. DXP and MEP are early metabolites of the MEP pathway, and their concentration in the chloroplast depends on the activity of the MEP pathway (Fig. 6). The amplitude of temperature dependence shown in Fig. 2 deviated from the profile predicted by default G-93.
Given that the amplitude of the temperature dependence of isoprene emission is linked to the flux of the MEP pathway, the higher amplitude of tropical tree may reflect the higher activity of this pathway under given growth conditions of the tropical area. The leaf-level isoprene emission over a short time scale is regulated by isoprene synthase and substrate supply (Monson and Fall 1989; Monson et al. 1992; Sharkey et al. 1996). Therefore, it is unlikely that the isoprene synthase level significantly changed in the short time scale of measurement in this study. Furthermore, no significant difference was noted in the isoprene synthase level between sunlit and shaded leaves of all tree species (Fig. 5), as was the case for poplar (Vickers et al. 2010). Thus, the shift of the G-93 parameter in this study may be primarily explained by the substrate availability, suggesting that the substrate concentration increased more steeply with temperature than predicted by default G-93. Alternatively, enzyme kinetics of isoprene synthase from tropical trees differs from that of temperate plants, and their higher affinity toward substrate DMADP may explain the steeper increase in isoprene emission as proposed in our previous studies (Higa et al. 2018; Mutanda et al. 2016a).
The CL parameter α plotted close to photosynthesis parameter ɸ defines the initial slope of light dependence of photosynthesis, with a correlation coefficient of 0.878 (p = 0.004) (Table 3). Thus, the CL formula of G-93 and the rectangular formula of photosynthesis are hyperbolic functions, and the light response curves of these two functions depict similar shapes as illustrated in Figs. 2 and 4. Therefore, it seems understandable that these two parameters are plotted close to each other in the same cluster.
Interestingly, the BEF was closely related to both the photon flux in the past 10 days of emission measurements (r = 0.878, p = 0.004), maximal photosynthesis rate (r = 0.832, p = 0.010), and half-saturation points (r = 0.855, p = 0.006). Photon flux is the principal driving force for isoprene emission and may influence emissions over a long-term scale. This could explain the weather effect over the past 48 and 240 h have been incorporated into the MEGAN isoprene emission model (Guenther et al. 2006). However, MEGAN is a canopy-level emission model for estimating the emission rate of isoprene and other biogenic volatile organic compounds into the atmosphere from terrestrial vegetation. It scales up leaf- or branch-level emission formula and incorporates the extended G-95 formula as the leaf-level emission model (Arneth et al. 2008). It has been reported that the light and temperature conditions of the past days resulted in large variations from the prediction via the G-93 algorithm (Petron et al. 2001; Sharkey et al. 1999). The MEGAN model considers leaf-level light intensity and temperature of past 24 and 240 h. However, there is no rationale for the 24 and 240 h, and these past time frames for weather effect were based on the few available studies at the time (Geron et al. 2000; Petron et al. 2001). Our results depicted in Fig. 9 may imply that air temperature of up to the past 5 days (including the measurement day) and photon flux averaged more than the past 7 days may better improve the predictions for isoprene emission. The weather effect of the past days may differ between temperate and tropical species and between species in the same ecosystem. Sharkey et al. observed a linear correlation between the basal emission rate and average temperature for the 2 days before the emission measurement (Sharkey et al. 1999). The correlation coefficient decreased with the past days, as observed with the past temperature effect on CT2 (Fig. 9a). Sharkey et al. further reported an improvement of correlation by including the daily PAR dose into the prediction. However, it was difficult to separate the effect of temperature and light intensity in their study because these two parameters often correlate.
Given the small number of observations (n = 8) in this experiment, a more systematic and expanded study is needed to test the weather effect on isoprene emission further. Moreover, there is growing evidence that tropical trees emit trace gases, such as methyl butanol and dimethyl sulfide (Jardine et al. 2015, 2013), that might interfere with chemiluminescence analysis. However, the emission of these gases was two orders of magnitude less compared to isoprene. Furthermore, the selectivity of isoprene measurement relative to many alkenes and non-alkenes, including dimethyl sulfide, is considered good in practice because isoprene reacted rapidly and emitted chemiluminescence effectively (Hills and Zimmerman 1990). Thus, the relative response factors of alkene and non-alkene compounds are less than 0.19 against 1 for isoprene. It is therefore unlikely that isoprene measurement in this study was significantly interfered with by another trace gas. Nevertheless, an assessment of interference by other trace gases in individual tree species would further improve the optimization of the isoprene emission parameters. The refinement of the approaches of our current study may give more insight into the environmental control of isoprene emission and contribute to improved prediction of regional emissions.
References
Affek HP, Yakir D (2002) Protection by isoprene against singlet oxygen in leaves. Plant Physiol 129:269–277
Alves EG, Harley P, Goncalves JFD, Moura CED, Jardine K (2014) Effects of light and temperature on isoprene emission at different leaf developmental stages of Eschweilera coriacea in central Amazon. Acta Amazon 44:9–18
Arneth A, Monson RK, Schurgers G, Niinemets Ü, Palmer PI (2008) Why are estimates of global terrestrial isoprene emissions so similar (and why is this not so for monoterpenes)? Atmos Chem Phys 8:4605–4620
Arntz HR, Willich SN, Schreiber C, Bruggemann T, Stern R, Schultheiss HP (2000) Diurnal, weekly and seasonal variation of sudden death—population-based analysis of 24 061 consecutive cases. Eur Heart J 21:315–320
Banerjee A, Wu Y, Banerjee R, Li Y, Yan HG, Sharkey TD (2013) Feedback inhibition of deoxy-d-xylulose-5-phosphate synthase regulates the methylerythritol 4-phosphate pathway. J Biol Chem 288:16926–16936
Benjamin MT, Sudol M, Bloch L, Winer AM (1996) Low-emitting urban forests: A taxonomic methodology for assigning isoprene and monoterpene emission rates. Atmospheric Environment 30:1437–1452
Bruggemann N, Schnitzler JP (2002) Diurnal variation of dimethylallyl diphosphate concentrations in oak (Quercus robur) leaves. Physiol Plant 115:190–196
Causton DR, Dale MP (1990) The monomolecular and rectangular hyperbola as empirical models of the response of photosynthetic rate to photon flux density, aith application to three Veronica species. Ann Bot 65:389–394
Chang C-C, Chen T-Y, Lin C-Y, Yuan C-S, Liu S-C (2005) Effects of reactive hydrocarbons on ozone formation in southern Taiwan. Atmos Environ 39:2867–2878
Fehsenfeld F, Calvert J, Fall R, Goldan P, Guenther AB, Hewitt CN, Lamb B, Liu S, Trainer M, Wesberg H (1992) Emissions of volatile organic compounds, from vegetation and the implications for atmospheric chemistry. Glob Biogeochem Cycles 96:389–430
Galan AB, Rodriguez M, de Juana S, Dominguez-Rodrigo M (2009) A new experimental study on percussion marks and notches and their bearing on the interpretation of hammerstone-broken faunal assemblages. J Archaeol Sci 36:776–784
Geron C, Guenther A, Sharkey T, Arnts RR (2000) Temporal variability in basal isoprene emission factor. Tree Physiol 20:799–805
Geron C, Guenther A, Greenberg J, Loescher HW, Clark D, Baker B (2002) Biogenic volatile organic compound emissions from a lowland tropical wet forest in Costa Rica. Atmos Environ 36:3793–3802
Guenther AE, Zimmerman PR, Harley PC, Monson RK, Fall R (1993) Isoprene and monoterpene emssion rate variability: model evaluations and sensitivity. J Geophys Res 98:12609–12617
Guenther A, Hewitt CN, Erickson D, Fall R, Geron C, Graedel T, Harley P, Klinger L, Lerdau M, Mckay WA, Pierce T, Scholes B, Steinbrecher R, Tallamraju R, Taylor J, Zimmerman P (1995) A global-model of natural volatile organic-compound emissions. J Geophys Res Atmos 100:8873–8892
Guenther A, Otter L, Zimmerman P, Greenberg J, Scholes R, Scholes M (1996) Biogenic hydrocarbon emissions from southern African savannas. J Geophys Res Atmos 101:25859–25865
Guenther A, Karl T, Harley P, Wiedinmyer C, Palmer PI, Geron C (2006) Estimates of global terrestrial isoprene emissions using MEGAN (model of emissions of gases and aerosols from nature). Atmos Chem Phys 6:3181–3210
Gupta S, Mishra K, Surolia A, Banerjee K (2011) Suppressor of cytokine signalling-6 promotes neurite outgrowth via JAK2/STAT5-mediated signalling pathway, involving negative feedback inhibition. PLoS ONE 6:e26674
Harley P, Guenther A, Zimmerman P (1996) Effects of light, temperature and canopy position on net photosynthesis and isoprene emission from sweetgum (Liquidambar styraciflua) leaves. Tree Physiol 16:25–32
Harley P, Guenther A, Zimmerman P (1997) Environmental controls over isoprene emission in deciduous oak canopies. Tree Physiol 17:705–714
He C, Murray F, Lyons T (2000) Monoterpene and isoprene emissions from 15 Eucalyptus species in Australia. Atmos Environ 34:645–655
Higa T, Parveen S, Mutanda I, Iqbal MA, Inafuku M, Hashimoto F, Oku H (2018) Evaluation of isoprene emission rates of tropical trees by an iterative optimization procedure for G-93 parameters. Atmos Environ 192:209–217
Hills AJ, Zimmerman PR (1990) Isoprene measurement by ozone-induced chemiluminescence. Anal Chem 62:1055–1060
Jardine KJ, Meyers K, Abrell L, Alves EG, Serrano AM, Kesselmeier J, Karl T, Guenther A, Chambers JQ, Vickers C (2013) Emissions of putative isoprene oxidation products from mango branches under abiotic stress. J Exp Bot 64:3697–3709
Jardine K, Yanez-Serrano AM, Williams J, Kunert N, Jardine A, Taylor T, Abrell L, Artaxo P, Guenther A, Hewitt CN, House E, Florentino AP, Manzi A, Higuchi N, Kesselmeier J, Behrendt T, Veres PR, Derstroff B, Fuentes JD, Martin ST, Andreae MO (2015) Dimethyl sulfide in the Amazon rain forest. Glob Biogeochem Cycles 29:19–32
Kim JC, Kim KJ, Kim DS, Han JS (2005) Seasonal variations of monoterpene emissions from coniferous trees of different ages in Korea. Chemosphere 59:1685–1696
Lantz AT, Allman J, Weraduwage SM, Sharkey TD (2019) Isoprene: new insights into the control of emission and mediation of stress tolerance by gene expression. Plant Cell Environ 42:2808–2826
Lerdau M, Keller M (1997) Controls on isoprene emission from trees in a subtropical dry forest. Plant Cell Environ 20:569–578
Li JJ, Wang GH, Wu C, Cao C, Ren YQ, Wang JY, Li J, Cao JJ, Zeng LM, Zhu T (2018) Characterization of isoprene-derived secondary organic aerosols at a rural site in North China Plain with implications for anthropogenic pollution effects. Sci Rep Uk 8:1–10
Loreto F, Fineschi S (2015) Reconciling functions and evolution of isoprene emission in higher plants. New Phytol 206:578–582
Loreto F, Pinelli P, Brancaleoni E, Ciccioli P (2004) C-13 labeling reveals chloroplastic and extrachloroplastic pools of dimethylallyl pyrophosphate and their contribution to isoprene formation. Plant Physiol 135:1903–1907
Meeningen Y, Schurgers G, Rinnan R, Holst T (2016) BVOC emissions from English oak (Quercus robur) and European beech (Fagus sylvatica) along a latitudinal gradient. Biogeosciences 13:6067–6080
Monson RK, Fall R (1989) Isoprene emission from aspen leaves : influence of environment and relation to photosynthesis and photorespiration. Plant Physiol 90:267–274
Monson RK, Jaeger CH, Adams WW, Driggers EM, Silver GM, Fall R (1992) Relationships among isoprene emission rate, photosynthesis, and isoprene synthase activity as influenced by temperature. Plant Physiol 98:1175–1180
Monson RK, Harley PC, Litvak ME, Wildermuth M, Guenther A, Zimmerman PR (1994) Evironmental and developmental controls over hte seasonal pattern of isoprene emission from aspen leaves. Oecologia 99:260–270
Mutanda I, Inafuku M, Iwasaki H, Saitoh S, Fukuta M, Watanabe K, Oku H (2016a) Parameterization of G-93 isoprene emission formula for tropical trees Casuarina equisetifolia and Ficus septica. Atmos Environ 141:287–296
Mutanda I, Inafuku M, Saitoh S, Iwasaki H, Fukuta M, Watanabe K, Oku H (2016b) Temperature controls on the basal emission rate of isoprene in a tropical tree Ficus septica: exploring molecular regulatory mechanisms. Plant Cell Environ 39:2260–2275
Oku H, Inafuku M, Takamine T, Nagamine M, Saitoh S, Fukuta M (2014) Temperature threshold of isoprene emission from tropical trees, Ficus virgata and Ficus septica. Chemosphere 95:268–273
Oku H, Inafuku M, Ishikawa T, Takamine T, Ishmael M, Fukuta M (2015) Molecular cloning and biochemical characterization of isoprene synthases from the tropical trees Ficus virgata, Ficus septica, and Casuarina equisetifolia. J Plant Res 128:849–861
Padhy PK, Varshney CK (2005) Emission of volatile organic compounds (VOC) from tropical plant species in India. Chemosphere 59:1643–1653
Parveen S, Harun-Ur-Rashid M, Inafuku M, Iwasaki H, Oku H (2019a) Molecular regulatory mechanism of isoprene emission under short-term drought stress in the tropical tree Ficus septica. Tree Physiol 39:440–453
Parveen S, Lqbal MA, Mutanda I, Harun-Ur-Rashid M, Inafuku M, Oku H (2019b) Plant hormone effects on isoprene emission from tropical tree in Ficus septica. Plant Cell Environ 42:1715–1728
Petron G, Harley P, Greenberg J, Guenther A (2001) Seasonal temperature variations influence isoprene emission. Geophys Res Lett 28:1707–1710
Poisson N, Kanakidou M, Crutzen PJ (2000) Impact of non-methane hydrocarbons on tropospheric chemistry and the oxidizing power of the global troposphere: 3-dimensional modelling results. J Atmos Chem 36:157–230
Reich PB, Amundson RG (1985) Ambient levels of ozone reduce net photosynthesis in tree and crop species. Science 230:566–570
Rinne HJI, Guenther AB, Greenberg JP, Harley PC (2002) Isoprene and monoterpene fluxes measured above Amazonian rainforest and their dependence on light and temperature. Atmos Environ 36:2421–2426
Rodriguez-Concepcion M, Boronat A (2015) Breaking new ground in the regulation of the early steps of plant isoprenoid biosynthesis. Curr Opin Plant Biol 25:17–22
Runeckles VC, Chevone BI (1992) Crop responses to ozone. In: Lefohn AS (ed) Surface level ozone exposures and their effects on vegetation. Lewis Publishers, Chelsea, pp 189–270
Sharkey TD, Monson RK (2014) The future of isoprene emission from leaves, canopies and landscapes. Plant Cell Environ 37:1727–1740
Sharkey TD, Singsaas EL, Vanderveer PJ, Geron C (1996) Field measurements of isoprene emission from trees in response to temperature and light. Tree Physiol 16:649–654
Sharkey TD, Singsaas EL, Lerdau MT, Geron CD (1999) Weather effects on isoprene emission capacity and applications in emissions algorithms. Ecol Appl 9:1132–1137
Squire OJ, Archibald AT, Griffiths PT, Jenkin ME, Smith D, Pyle JA (2015) Influence of isoprene chemical mechanism on modelled changes in tropospheric ozone due to climate and land use over the 21st century. Atmos Chem Phys 15:5123–5143
Trainer M, Williams EJ, Parrish DD, Buhr MP, Allwine EJ, Westberg HH, Fehsenfeld FC, Liu FC (1987) Models and observations of the impact of natural hydrocarbons on rural ozone. Nature 329:705–707
Vickers CE, Possell M, Nicholas Hewitt C, Mullineaux PM (2010) Genetic structure and regulation of isoprene synthase in Poplar (Populus spp.). Plant Mol Biol 73:547–558
Wright LP, Rohwer JM, Ghirardo A, Hammerbacher A, Ortiz-Alcaide M, Raguschke B, Schnitzler JP, Gershenzon J, Phillips MA (2014) Deoxyxylulose 5-phosphate synthase controls flux through the methylerythritol 4-phosphate pathway in Arabidopsis. Plant Physiol 165:1488–1504
Xiaoshan Z, Yujing M, Wenzhi S, Yahui Z (2000) Seasonal variations of isoprene emissions from deciduous trees. Atmos Environ 34:3027–3032
Acknowledgements
This study was funded in part by the Japan Society for Promotion of Science (KAKENHI# 19H03089).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oku, H., Iwai, S., Uehara, M. et al. Growth condition controls on G-93 parameters of isoprene emission from tropical trees. J Plant Res 134, 1225–1242 (2021). https://doi.org/10.1007/s10265-021-01344-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-021-01344-x