Assessment of Capsicum annuum L. Grown in Controlled and Semi-Controlled Environments Irrigated with Greywater Treated by Floating Wetland Systems
Abstract
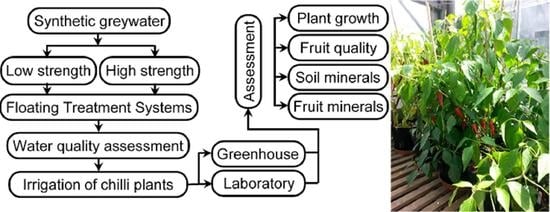
:1. Introduction
2. Materials and Methods
2.1. Operational Design of Floating Treatment Wetlands
2.2. Material Selection and Chilli Planting Processes
2.3. Growth Environment Monitoring and Recording
2.4. Justification of Chilli Plant Selection
2.5. Water Quality, Soil, and Chilli Fruit Analysis
2.6. Data Statistical Analysis
3. Results and Discussion
3.1. Irrigation Water Quality
3.2. Growth Environmental Conditions
3.3. Growth Monitoring
3.4. Chilli Fruit Production, Quality, and Classification
3.5. Accumulated Trace Elements in Soil
3.6. Accumulated Trace Elements in Chilli Fruits
4. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. User Manual for Irrigation with Treated Wastewater; Food and Agriculture Organization (FAO) of the United Nations, Regional Office for Near East: Cairo, Egypt, 2003. [Google Scholar]
- WHO. Guidelines for the Safe Use of Wastewater, Excreta and Greywater: Wastewater Use in Agriculture; World Health Organization (WHO): Geneva, Switzerland, 2006; Volume 2. [Google Scholar]
- Almuktar, S.A.A.A.N.; Abed, S.N.; Scholz, M. Contaminations of Soil and Two Capsicum annuum Generations Irrigated by Reused Urban Wastewater Treated by Different Reed Beds. Int. J. Environ. Res. Public Health 2018, 15, 1776. [Google Scholar] [CrossRef] [Green Version]
- Almuktar, S.A.A.A.N.; Abed, S.N.; Scholz, M. Wetlands for wastewater treatment and subsequent recycling of treated effluent: A review. Environ. Sci. Pollut. Res. 2018, 25, 23595–23623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DESA-UN. World Population Prospects 2019: Highlights; Report (ST/ESA/SER.A/423); United Nations, Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2019. [Google Scholar]
- Alcamo, J.; Döll, P.; Kaspar, F.; Stefan, S. Global Change and Global Scenarios of Water Use and Availability: An Application of WaterGAP 1.0; Centre for Environmental Systems Research, University of Kassel: Kassel, Germany, 1997. [Google Scholar]
- Alcamo, J.; Henrichs, T.; Rösch, T. World Water in 2025: Global Modelling and Scenario Analysis for the World Commission on Water for the 21st Century; Centre for Environmental Systems Research, University of Kassel: Kassel, Germany, 2000. [Google Scholar]
- Scheierling, S.M.; Bartone, C.R.; Mara, D.D.; Drechsel, P. Towards an agenda for improving wastewater use in agriculture. Water Int. 2011, 36, 420–440. [Google Scholar] [CrossRef]
- WWAP-UN. The United Nations World Water Development Report 4: Managing Water Under Uncertainty and Risk; UNESCO, World Water Assessment Programme (WWAP): Paris, France, 2012. [Google Scholar]
- WHO. Health Guidelines for the Use of Wastewater in Agriculture and Aquaculture; Technical Report Series No. 77; World Health Organization (WHO): Geneva, Switzerland, 1989. [Google Scholar]
- USEPA. Guidelines for Water Reuse; Report (EPA/600/R-12/618); United States Environmental Protection Agency (USEPA): Washington, DC, USA, 2012. [Google Scholar]
- Cirelli, G.L.; Consoli, S.; Licciardello, F.; Aiello, R.; Giuffrida, F.; Leonardi, C. Treated municipal wastewater reuse in vegetable production. Agric. Water Manag. 2012, 104, 163–170. [Google Scholar] [CrossRef]
- Willer, H.; Lernoud, J. The World of Organic Agriculture. Statistics and Emerging Trends; Research Institute of Organic Agriculture (FiBL), Frick, and IFOAM–Organics International: Bonn, Germany, 2019. [Google Scholar]
- Weissengruber, L.; Möller, K.; Puschenreiter, M.; Friedel, J.K. Long-term soil accumulation of potentially toxic elements and selected organic pollutants through application of recycled phosphorus fertilizers for organic farming conditions. Nutr. Cycl. Agroecosyst. 2018, 110, 427–449. [Google Scholar] [CrossRef] [Green Version]
- UNESCO. Water for People-Water for Life: A Joint Report by the Twenty-Three United Nations Agencies Concerned with Freshwater; United Nations Educational, Scientific and Cultural Organization (UNESCO): Barcelona, Spain, 2003. [Google Scholar]
- Shuval, H.I.; Adin, A.; Fattal, B.; Rawitz, E.; Yekutiel, P. Wastewater Irrigation in Developing Countries: Health Effects and Technical Solutions; Technical Paper No. 51; World Bank: Washington, DC, USA, 1986. [Google Scholar]
- Dalahmeh, S.; Baresel, C. Reclaimed Wastewater Use Alternatives and Quality Standards from Global to Country Perspective: Spain versus Abu Dhabi Emirate; IVL Swedish Environmental Research Institute: Uppsala/Stockholm, Sweden, 2014. [Google Scholar]
- Toze, S. Reuse of effluent water–Benefits and risks. Agric. Water Manag. 2006, 80, 147–159. [Google Scholar] [CrossRef] [Green Version]
- Nolde, E. Greywater reuse systems for toilet-flushing in multi-storey buildings-over ten years’ experience in Berlin. Urban Water 1999, 1, 275–284. [Google Scholar] [CrossRef]
- Christova-Boal, D.; Eden, R.E.; McFarlane, S. An investigation into greywater reuse for urban residential properties. Desalination 1996, 106, 391–397. [Google Scholar] [CrossRef]
- Eriksson, E.; Auffarth, K.; Henze, M.; Ledin, A. Characteristics of grey wastewater. Urban Water 2002, 4, 85–104. [Google Scholar] [CrossRef]
- Al-Jayyousi, O.R. Greywater reuse: Towards sustainable water management. Desalination 2003, 156, 181–192. [Google Scholar] [CrossRef]
- Edogbo, B.; Okolocha, E.; Maikai, B.; Aluwong, T.; Uchendu, C. Risk Analysis of Heavy Metal Contamination in Soil, Vegetables and Fish around Challawa Area in Kano State, Nigeria. Sci. Afr. 2020, 17, e00281. [Google Scholar] [CrossRef]
- Hussain, M.I.; Qureshi, A.S. Health risks of heavy metal exposure and microbial contamination through consumption of vegetables irrigated with treated wastewater at Dubai, UAE. Environ. Sci. Pollut. Res. 2020, 27, 11213–11226. [Google Scholar] [CrossRef]
- Osmani, M.; Bani, A.; Hoxha, B. Heavy Metals and Ni Phytoextraction in the Metallurgical Area Soils in Elbasan. Albanian J. Agric. Sci. 2015, 14, 414–419. [Google Scholar]
- Mkhinini, M.; Boughattas, I.; Alphonse, V.; Livet, A.; Gıustı-Mıller, S.; Bannı, M.; Bousserrhıne, N. Heavy metal accumulation and changes in soil enzymes activities and bacterial functional diversity under long-term treated wastewater irrigation in East Central region of Tunisia (Monastir governorate). Agric. Water Manag. 2020, 235, 106–150. [Google Scholar] [CrossRef]
- FAO/WHO. Codex Committee on Contaminants in Foods 10th Session; Working Document for Information and Use in Discussions Related to Contaminants and Toxins in the GSCTFF Volume 4; Food and Agriculture Organization, Rome, Italy 2016. Available online: http://www.fao.org/fao-who-codexalimentarius (accessed on 9 August 2021).
- Hadayat, N.; De Oliveira, L.M.; Da Silva, E.; Han, L.; Hussain, M.; Liu, X.; Ma, L.Q. Assessment of trace metals in five most-consumed vegetables in the US: Conventional vs. organic. Environ. Pollut. 2018, 243, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Awashthi, S.K. Prevention of Food Adulteration Act No 37 of 1954; Central and State Rules as Amended for 1999; Ashoka Law House: New Delhi, India, 2000. [Google Scholar]
- NHFPC. GB 2762-2012 China Food Safety National Standard for Maximum Levels of Contaminants in Foods; NHFPC-National Health and Family Planning of People’s Republic of China: Beijing, China, 2012. [Google Scholar]
- European Union (EU). Heavy Metals in Wastes, European Commission on Environment; European Union: Brussels, Belgium, 2002; Available online: http://ec.europa.eu/environment/waste/studies/pdf/heavymetalsreport.pdf (accessed on 9 August 2021).
- WHO. Permissible Limits of Heavy Metals in Soil and Plants; World Health Organization (WHO): Geneva, Switzerland, 1996. [Google Scholar]
- Douay, F.; Pelfrêne, A.; Planque, J.; Fourrier, H.; Richard, A.; Roussel, H.; Girondelot, B. Assessment of potential health risk for inhabitants living near a former lead smelter. Part 1: Metal concentrations in soils, agricultural crops, and home grown vegetables. Environ. Monit. Assess. 2013, 185, 3665–3680. [Google Scholar] [CrossRef]
- Hu, J.; Wu, F.; Wu, S.; Cao, Z.; Lin, X.; Wong, M.H. Bioaccessibility, dietary exposure and human risk assessment of heavy metals from market vegetables in Hong Kong revealed with an in vitro gastrointestinal model. Chemosphere 2013, 91, 455–461. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, W.T.; Zhou, X.; Liu, L.; Gu, J.F.; Wang, W.L.; Zou, J.L.; Tian, T.; Peng, P.Q.; Liao, B.H. Accumulation of heavy metals in vegetable species planted in contaminated soils and the health risk assessment. Int. J. Environ. Res. Public Health 2016, 13, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigdeli, M.; Seilsepour, M. Investigation of metals accumulation in some vegetables irrigated with waste water in Shahre Rey-Iran and toxicological implications. Am.-Eurasian J. Agric. Environ. Sci. 2008, 4, 86–92. [Google Scholar]
- Shaheen, N.; Irfan, N.M.; Khan, I.N.; Islam, S.; Islam, M.S.; Ahmed, M.K. Presence of heavy metals in fruits and vegetables: Health risk implications in Bangladesh. Chemosphere 2016, 152, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. 2008, 152, 686–692. [Google Scholar] [CrossRef]
- Khan, K.; Lu, Y.; Khan, H.; Ishtiaq, M.; Khan, S.; Waqas, M.; Wei, L.; Wang, T. Heavy metals in agricultural soils and crops and their health risks in Swat District, northern Pakistan. Food Chem. Toxicol. 2013, 58, 449–458. [Google Scholar] [CrossRef]
- Scholz, M.; Lee, B.H. Constructed wetlands: A review. Int. J. Environ. Stud. 2005, 62, 421–447. [Google Scholar] [CrossRef]
- Vymazal, J. Removal of nutrients in various types of constructed wetlands. Sci. Total Environ. 2007, 380, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M. Sustainable Water Treatment: Engineering Solutions for a Variable Climate; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Brix, H. Use of constructed wetlands in water pollution control: Historical development, present status, and future perspectives. Water Sci. Technol. 1994, 30, 209–224. [Google Scholar] [CrossRef] [Green Version]
- Vymazal, J. Horizontal sub-surface flow and hybrid constructed wetlands systems for wastewater treatment: Review. Ecol. Eng. 2005, 25, 478–490. [Google Scholar] [CrossRef]
- Scholz, M. Wetland Systems–Storm Water Management Control; Springer: Berlin, Germany, 2010. [Google Scholar]
- Borne, K.E.; Fassman-Beck, E.A.; Tanner, C.C. Floating treatment wetland retrofit to improve stormwater pond performance for suspended solids, copper and zinc. Ecol. Eng. 2013, 54, 173–182. [Google Scholar] [CrossRef]
- Wang, C.Y.; Sample, D.J. Assessment of the nutrient removal effectiveness of floating treatment wetlands applied to urban retention ponds. J. Environ. Manag. 2014, 137, 23–35. [Google Scholar] [CrossRef]
- Keizer-Vlek, H.E.; Verdonschot, P.F.M.; Verdonschot, R.C.M.; Dekkers, D. The contribution of plant uptake to nutrient removal by floating treatment wetlands. Ecol. Eng. 2014, 73, 684–690. [Google Scholar] [CrossRef]
- Rezania, S.; Taib, S.M.; Din, M.F.M.; Dahalan, F.A.; Kamyab, H. Comprehensive review on phytotechnology: Heavy metals removal by diverse aquatic plants species from wastewater. J. Hazard. Mater. 2016, 318, 587–599. [Google Scholar] [CrossRef]
- Abed, S.N.; Scholz, M. Chemical simulation of greywater. Environ. Technol. 2016, 37, 1631–1646. [Google Scholar] [CrossRef] [PubMed]
- Abed, S.N.; Almuktar, S.A.A.A.N.; Scholz, M. Phytoremediation performance of floating treatment wetlands with pelletized mine water sludge for synthetic greywater treatment. J. Environ. Health Sci. Eng. 2019, 372, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Abed, S.N.; Almuktar, S.A.A.A.N.; Scholz, M. Remediation of synthetic greywater in mesocosm-scale floating treatment wetlands. Ecol. Eng. 2017, 102, 303–319. [Google Scholar] [CrossRef]
- Abed, S.N.; Almuktar, S.A.A.A.N.; Scholz, M. Treatment of contaminated greywater using pelletised mine water sludge. J. Environ. Manag. 2017, 197, 10–23. [Google Scholar] [CrossRef]
- Almuktar, S.A.A.A.N.; Scholz, M.; Al-Isawi, R.; Sani, A. Recycling of domestic wastewater treated by vertical-flow wetlands for watering of vegetables. Water Pract. Technol. 2015, 10, 445–464. [Google Scholar] [CrossRef]
- Nickels, J. Growing Chillies—A Guide to the Domestic Cultivation of Chilli Plants; Jason Nickels: London, UK, 2012. [Google Scholar]
- Jones, J.B., Jr. Instructions for Growing Tomatoes in the Garden and Greenhouse; GroSystems: Anderson, SC, USA, 2013. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association (APHA), American Water Works Association, and Water and Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- USEPA. SW–846: Test method 6010D: Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES); Revision 4; United States Environmental Protection Agency (USEPA): Washington, DC, USA, 2014. [Google Scholar]
- USEPA. Method 200.7: Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma-Atomic Emission Spectrometry; Revision 4.4; United States Environmental Protection Agency (USEPA): Washington, DC, USA, 1994. [Google Scholar]
- Chary, N.S.; Kamala, C.T.; Raj, D.S.S. Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol. Environ. Saf. 2008, 69, 513–524. [Google Scholar] [CrossRef]
- USEPA. Method 3050B: Acid Digestion of Sediments, Sludges, and Soils; Revision 2; United States Environmental Protection Agency (USEPA): Washington, DC, USA, 1996. [Google Scholar]
- Plank, C.O. Plant Analysis Reference Procedures for the Southern Region of the United States.; Southern Cooperative Series Bulletin number 368; University of Georgia: Athens, GA, USA, 1992. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Kasuya, E. Mann-Whitney U test when variances are unequal. Anim. Behav. 2001, 61, 1247–1249. [Google Scholar] [CrossRef] [Green Version]
- Stoline, M.R. The status of multiple comparisons: Simultaneous estimation of all pairwise comparisons in one-way ANOVA designs. Am. Stat. 1981, 35, 134–141. [Google Scholar]
- Almuktar, S.A.A.A.N.; Scholz, M. Mineral and biological contamination of soil and Capsicum annuum irrigated with recycled domestic wastewater. Agric. Water Manag. 2016, 167, 95–109. [Google Scholar] [CrossRef]
- Decreto Ministeriale. Regulating Technical Standards for Wastewater Reuse; Decreto Ministeriale: Rome, Italy, 2003; Volume 185. [Google Scholar]
- Bar-Tal, A.; Aloni, B.; Karni, L.; Rosenberg, R. Nitrogen nutrition of greenhouse pepper. II. Effects of nitrogen concentration and NO3:NH4 ratio on growth, transpiration, and nutrient uptake. HortScience 2001, 36, 1252–1259. [Google Scholar] [CrossRef]
- Millaleo, R.; Reyes-Díaz, M.; Ivanov, A.; Mora, M.; Alberdi, M. Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanisms. J. Soil Sci. Plant Nutr. 2010, 10, 470–481. [Google Scholar] [CrossRef] [Green Version]
- McCauly, A.; Jones, C.; Jacobsen, J. Plant Nutrient Functions and Deficiency and Toxicity Symptoms; Nutrient Management Module No. 9; Montana State University: Bozeman, MT, USA, 2011. [Google Scholar]
- BOE. Royal Decree 140/2003 That Establish Health Criteria of Water Quality for Human Consumption; Boletín Oficial de Estado (BOE) No 45, 21/02/2003; BOE: Madrid, Spain, 2003. [Google Scholar]
- Ewers, U. Standards, guidelines and legislative regulations concerning metals and their compounds. In Metals and Their Compounds in the Environment: Occurrence, Analysis and Biological Relevance; Merian, E., Ed.; VCH: Weinheim, Germany, 1991; pp. 458–468. [Google Scholar]
- NJDEP. Soil Cleanup Criteria; Proposed Cleanup Standards for Contaminated Sites, NJAC 7:26D; New Jersey Department of Environmental Protection: Tuckerton, NJ, USA, 1996. [Google Scholar]
- DPR-EGASPIN. Environmental Guidelines and Standards for the Petroleum Industry in Nigeria (EGASPIN); Department of Petroleum Resources: Lagos, Nigeria, 2002. [Google Scholar]
- Almuktar, S.A.A.A.N.; Scholz, M.; Al-Isawi, R.H.K.; Sani, A. Recycling of domestic wastewater treated by vertical-flow wetlands for irrigating Chillies and Sweet Peppers. Agric. Water Manag. 2015, 149, 1–22. [Google Scholar] [CrossRef]
- Pinto, U.; Maheshwari, B.L.; Grewal, H.S. Effects of greywater irrigation on plant growth, water use and soil properties. Resour. Conserv. Recycl. 2010, 54, 429–435. [Google Scholar] [CrossRef]
- García-Delgado, C.; Eymar, E.; Contreras, J.I.; Segura, M.L. Effects of fertigation with purified urban wastewater on soil and pepper plant (Capsicum annuum L.) production, fruit quality and pollutant contents. Span. J. Agric. Res. 2012, 10, 209–221. [Google Scholar] [CrossRef] [Green Version]
- Al-Isawi, R.H.; Scholz, M.; Al-Faraj, F.A. Assessment of diesel-contaminated domestic wastewater treated by constructed wetlands for irrigation of chillies grown in a greenhouse. Environ. Sci. Pollut. Res. 2016, 23, 25003–25023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, R.M.; Rao, N.K.S. Photosynthesis and dry matter partitioning in three cultivars of Capsicum annuum L. grown at two temperature conditions. Photosynthetica 1989, 23, 21–26. [Google Scholar]
- Almuktar, S.A.A.A.N.; Abed, S.N.; Scholz, M. Recycling of domestic wastewater treated by vertical-flow wetlands for irrigation of two consecutive Capsicum annuum generations. Ecol. Eng. 2017, 107, 82–98. [Google Scholar] [CrossRef]
- Deli, J.; Tiessen, H. Interaction of temperature and light intensity on flowering of Capsicum frutescens var grossum cv. California Wonder. J. Am. Soc. Hortic. Sci. 1969, 94, 349–351. [Google Scholar]
- Al-Isawi, R.H.; Almuktar, S.A.A.A.N.; Scholz, M. Monitoring and assessment of treated river, rain, gully pot and grey waters for irrigation of Capsicum annuum. Environ. Monit. Assess. 2016, 188, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Z.; Li, Y.; Sun, Q.; Zhang, H. Trace elements in soils and selected agricultural plants in the Tongling mining area of China. Int. J. Environ. Res. Public Health 2018, 15, 202. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.K.; Agrawal, M.; Marshall, F. Heavy metal contamination in vegetables grown in wastewater irrigated areas of Varanasi, India. Bull. Environ. Contam. Toxicol. 2006, 77, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sharma, R.K.; Agrawal, M.; Marshall, F.M. Risk assessment of heavy metal toxicity through contaminated vegetables from waste water irrigated area of Varanasi, India. Trop. Ecol. 2010, 51, 375–387. [Google Scholar]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Int. Sch. Res. Net. ISRN Ecol. 2011, 2011, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Chemicals, H. Crop Guide: Peppers; Haifa Group: Haifa, Israel, 2019; Available online: https://www.haifa-group.com/articles/crop-guide-peppers/ (accessed on 9 August 2021).











| Treatment System | HRT | SGW | TW | Vegetation | Cement–Ochre | Plant Receiving the Effluent | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2-Day | 7-Day | HC | LC | With | Without | With | Without | |||
| T1 | ♦ | ♦ | ♦ | ♦ | P1 | |||||
| T2 | ♦ | ♦ | ♦ | ♦ | P2 | |||||
| T3 | ♦ | ♦ | ♦ | ♦ | P3 | |||||
| T4 | ♦ | ♦ | ♦ | ♦ | P4 | |||||
| T5 | ♦ | ♦ | ♦ | ♦ | P5 | |||||
| T6 | ♦ | ♦ | ♦ | ♦ | P6 | |||||
| T7 | ♦ | ♦ | ♦ | ♦ | P7 | |||||
| T8 | ♦ | ♦ | ♦ | ♦ | P8 | |||||
| T9 | ♦ | ♦ | ♦ | ♦ | P9 | |||||
| T10 | ♦ | ♦ | ♦ | ♦ | P10 | |||||
| T11 | ♦ | ♦ | ♦ | ♦ | P11 | |||||
| T12 | ♦ | ♦ | ♦ | ♦ | P12 | |||||
| T13 | ♦ | ♦ | ♦ | ♦ | P13 | |||||
| T14 | ♦ | ♦ | ♦ | ♦ | P14 | |||||
| T15 | ♦ | ♦ | ♦ | ♦ | P15 | |||||
| T16 | ♦ | ♦ | ♦ | ♦ | P16 | |||||
| C1 | ♦ | ♦ | ♦ | ♦ | P1c | |||||
| C2 | ♦ | ♦ | ♦ | ♦ | P2c | |||||
| C3 | ♦ | ♦ | ♦ | ♦ | P3c | |||||
| C4 | ♦ | ♦ | ♦ | ♦ | P4c | |||||
| Parameter [57] | Unit | Influent | 2-Day HRT (HC-SGW Effluent) | Influent | 2-Day HRT (LC-SGW Effluent) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HC-SGW | T1 | T2 | T3 | T4 | LC-SGW | T5 | T6 | T7 | T8 | ||
| pH | – | 8.4 ± 1.61 | 7.4 ± 1.09 | 8.8 ± 1.69 | 7.8 ± 1.37 | 8.7 ± 1.73 | 6.9 ± 0.48 | 7.0 ± 0.71 | 10.5 ± 1.12 | 7.5 ± 0.70 | 10.6 ± 0.99 |
| Redox potential | mV | −36.6 ± 74.22 | 8.1 ± 52.68 | −54.8 ± 83.66 | −3.0 ± 62.95 | −49.9 ± 83.61 | 34.1 ± 21.23 | 27.5 ± 32.18 | −137.4 ± 54.91 | 4.2 ± 30.40 | −143.5 ± 51.01 |
| Turbidity | NTU | 188.9 ± 47.22 | 175.9 ± 59.61 | 223.8 ± 97.40 | 192.1 ± 50.87 | 191.3 ± 84.41 | 22.9 ± 7.14 | 28.2 ± 37.09 | 39.2 ± 45.10 | 20.2 ± 14.20 | 35.6 ± 18.11 |
| Total suspended solids | mg/L | 317.0 ± 58.35 | 302.9 ± 75.19 | 422.5 ± 152.77 | 321.8 ± 56.68 | 337.4 ± 109.45 | 39.9 ± 15.94 | 41.7 ± 43.57 | 62.0 ± 49.93 | 30.0 ± 12.12 | 66.2 ± 36.63 |
| Electronic conductivity | µS/cm | 988.5 ± 196.09 | 987.4 ± 107.25 | 1174.5 ± 282.81 | 965.2 ± 106.68 | 1178.4 ± 264.41 | 164.6 ± 63.24 | 145.9 ± 30.41 | 371.5 ± 260.12 | 138.5 ± 23.26 | 344.5 ± 287.03 |
| Dissolved oxygen | mg/L | 10.5 ± 1.39 | 9.0 ± 1.03 | 9.0 ± 1.24 | 10.2 ± 0.73 | 10.0 ± 0.52 | 10.4 ± 1.24 | 9.3 ± 1.08 | 8.8 ± 0.87 | 10.5 ± 0.82 | 10.1 ± 0.73 |
| Colour | Pa/Co | 1587.8 ± 379.89 | 1525.6 ± 411.54 | 2150.8 ± 864.04 | 1527.6 ± 326.28 | 1935.6 ± 702.18 | 214.5 ± 64.07 | 183.7 ± 74.89 | 308.2 ± 134.65 | 164.5 ± 40.93 | 331.7 ± 119.34 |
| Temperature | °C | 16.9 ± 5.40 | 17.1 ± 4.92 | 17.4 ± 4.87 | 17.1 ± 4.75 | 17.2 ± 4.73 | 17.7 ± 4.58 | 17.0 ± 4.84 | 16.6 ± 4.55 | 16.0 ± 4.59 | 16.3 ± 4.24 |
| Biochemical oxygen demand | mg/L | 34.7 ± 12.99 | 17.7 ± 6.40 | 11.1 ± 5.89 | 14.7 ± 7.78 | 11.7 ± 7.71 | 17.6 ± 8.00 | 9.9 ± 5.49 | 5.4 ± 4.36 | 5.6 ± 3.60 | 4.4 ± 5.13 |
| Chemical oxygen demand | mg/L | 129.2 ± 34.68 | 96.3 ± 32.01 | 109.2 ± 24.38 | 106.6 ± 22.68 | 100.3 ± 21.08 | 28.9 ± 14.47 | 32.4 ± 14.55 | 29.6 ± 16.67 | 26.8 ± 6.18 | 24.0 ± 4.99 |
| Ammonia–nitrogen | mg/L | 0.4 ± 0.19 | 0.4 ± 0.21 | 0.4 ± 0.13 | 0.4 ± 0.16 | 0.4 ± 0.09 | 0.2 ± 0.22 | 0.1 ± 0.07 | 0.2 ± 0.14 | 0.09 ± 0.05 | 0.1 ± 0.04 |
| Nitrate–nitrogen | mg/L | 8.9 ± 6.38 | 14.1 ± 6.40 | 14.3 ± 5.02 | 9.4 ± 4.67 | 12.9 ± 7.03 | 1.3 ± 1.21 | 1.7 ± 1.13 | 0.4 ± 0.33 | 1.2 ± 0.71 | 0.6 ± 0.54 |
| Ortho-phosphate–phosphorus | mg/L | 59.1 ± 14.16 | 52.0 ± 14.87 | 21.1 ± 5.81 | 46.2 ± 10.74 | 19.5 ± 4.98 | 8.4 ± 4.36 | 7.6 ± 3.90 | 3.2 ± 1.16 | 7.0 ± 3.89 | 3.9 ± 1.25 |
| Element [58,59] | |||||||||||
| Aluminium (Al) | mg/L | 2.13 ± 0.869 | 1.54 ± 1.479 | 2.02 ± 1.624 | 2.41 ± 1.016 | 2.98 ± 2.087 | 0.52 ± 0.528 | 0.08 ± 0.054 | 1.07 ± 0.874 | 0.34 ± 0.180 | 0.76 ± 0.347 |
| Boron (B) | mg/L | 0.57 ± 0.068 | 0.53 ± 0.086 | 0.41 ± 0.079 | 0.54 ± 0.060 | 0.50 ± 0.078 | 0.14 ± 0.067 | 0.11 ± 0.010 | 0.09 ± 0.011 | 0.11 ± 0.009 | 0.10 ± 0.024 |
| Calcium (Ca) | mg/L | 36.08 ± 8.750 | 42.50 ± 4.561 | 81.39 ± 23.641 | 43.02 ± 2.411 | 104.13 ± 32.868 | 10.54 ± 0.853 | 11.51 ± 0.926 | 45.13 ± 11.676 | 11.25 ± 0.773 | 70.99 ± 33.166 |
| Cadmium (Cd) | mg/L | 7.36 ± 2.981 | 4.90 ± 2.730 | 4.10 ± 1.839 | 7.69 ± 1.064 | 7.14 ± 2.429 | 0.09 ± 0.056 | 0.04 ± 0.020 | 0.03 ± 0.019 | 0.05 ± 0.031 | 0.04 ± 0.030 |
| Chromium (Cr) | mg/L | 3.20 ± 0.918 | 2.48 ± 2.060 | 2.74 ± 2.021 | 3.76 ± 1.203 | 3.99 ± 1.806 | 0.04 ± 0.063 | 0.03 ± 0.036 | 0.03 ± 0.033 | 0.04 ± 0.049 | 0.05 ± 0.039 |
| Copper (Cu) | mg/L | 1.44 ± 0.435 | 0.95 ± 0.561 | 0.90 ± 0.375 | 1.45 ± 0.113 | 1.55 ± 0.308 | 0.16 ± 0.058 | 0.04 ± 0.029 | 0.04 ± 0.035 | 0.06 ± 0.049 | 0.05 ± 0.043 |
| Iron (Fe) | mg/L | 6.41 ± 2.476 | 4.31 ± 2.928 | 4.71 ± 2.744 | 6.35 ± 2.423 | 7.11 ± 2.934 | 0.21 ± 0.102 | 0.15 ± 0.118 | 0.21 ± 0.202 | 0.21 ± 0.157 | 0.48 ± 0.447 |
| Potassium (K) | mg/L | 60.16 ± 1.684 | 52.79 ± 1.322 | 54.03 ± 11.214 | 55.68 ± 4.486 | 60.47 ± 15.561 | 4.04 ± 0.448 | 3.40 ± 0.675 | 10.78 ± 10.185 | 3.87 ± 0.364 | 12.77 ± 15.139 |
| Magnesium (Mg) | mg/L | 17.16 ± 2.119 | 17.32 ± 1.296 | 11.01 ± 2.533 | 17.76 ± 1.392 | 13.33 ± 4.526 | 1.45 ± 0.191 | 1.36 ± 0.157 | 0.63 ± 0.310 | 1.35 ± 0.133 | 0.70 ± 0.336 |
| Manganese (Mn) | mg/L | 0.98 ± 0.257 | 0.48 ± 0.320 | 0.51 ± 0.255 | 1.19 ± 0.063 | 0.89 ± 0.396 | 0.17 ± 0.084 | 0.01 ± 0.012 | 0.04 ± 0.031 | 0.08 ± 0.056 | 0.08 ± 0.069 |
| Sodium (Na) | mg/L | 62.68 ± 14.538 | 58.54 ± 11.080 | 56.95 ± 9.494 | 58.19 ± 10.620 | 58.54 ± 11.630 | 14.32 ± 1.662 | 14.74 ± 1.282 | 15.90 ± 1.869 | 13.82 ± 1.175 | 15.35 ± 3.197 |
| Nickel (Ni) | mg/L | 0.05 ± 0.065 | 0.02 ± 0.019 | 0.02 ± 0.019 | 0.03 ± 0.018 | 0.03 ± 0.033 | 0.04 ± 0.065 | 0.004 ± 0.006 | 0.01 ± 0.010 | 0.01 ± 0.007 | 0.01 ± 0.012 |
| Zinc (Zn) | mg/L | 4.25 ± 1.500 | 2.86 ± 1.680 | 2.58 ± 1.114 | 4.30 ± 0.524 | 4.52 ± 0.961 | 0.21 ± 0.159 | 0.06 ± 0.066 | 0.04 ± 0.054 | 0.09 ± 0.083 | 0.07 ± 0.084 |
| Parameter [57] | Unit | Influent | 7-day HRT (HC-SGW effluent) | Influent | 7-day HRT (LC-SGW effluent) | ||||||
| HC-SGW | T9 | T10 | T11 | T12 | LC-SGW | T13 | T14 | T15 | T16 | ||
| pH | – | 8.4 ± 1.61 | 7.3 ± 0.82 | 9.8 ± 1.34 | 7.7 ± 1.21 | 9.8 ± 1.54 | 6.9 ± 0.48 | 6.9 ± 0.61 | 10.3 ± 1.33 | 7.5 ± 0.72 | 10.5 ± 1.05 |
| Redox potential | mV | −36.6 ± 74.22 | 12.2 ± 40.30 | −100.1 ± 66.45 | −4.4 ± 59.67 | −95.5 ± 88.21 | 34.1 ± 21.23 | 31.0 ± 28.12 | −130.8 ± 63.74 | 1.8 ± 33.00 | −131.3 ± 72.36 |
| Turbidity | NTU | 188.9 ± 47.22 | 154.8 ± 86.08 | 178.8 ± 98.79 | 185.7 ± 49.24 | 245.8 ± 96.29 | 22.9 ± 7.14 | 18.9 ± 11.05 | 25.1 ± 16.21 | 16.5 ± 7.27 | 40.9 ± 25.03 |
| Total suspended solids | mg/L | 317.0 ± 58.35 | 267.8 ± 110.05 | 342.9 ± 125.33 | 302.6 ± 61.44 | 423.4 ± 114.04 | 39.9 ± 15.94 | 27.7 ± 16.48 | 37.5 ± 15.62 | 25.0 ± 10.96 | 55.2 ± 24.85 |
| Electronic conductivity | µS/cm | 988.5 ± 196.09 | 1137.4 ± 471.09 | 1191.1 ± 343.72 | 1003.0 ± 306.88 | 1107.1 ± 299.47 | 164.6 ± 63.24 | 161.4 ± 42.91 | 306.8 ± 118.32 | 144.0 ± 32.28 | 290.2 ± 135.74 |
| Dissolved oxygen | mg/L | 10.5 ± 1.39 | 8.8 ± 0.89 | 8.3 ± 1.03 | 10.5 ± 0.91 | 9.8 ± 1.19 | 10.4 ± 1.24 | 9.3 ± 1.24 | 8.7 ± 0.94 | 11.0 ± 1.11 | 10.1 ± 0.84 |
| Colour | Pa/Co | 1587.8 ± 379.89 | 1448.1 ± 647.98 | 1593.5 ± 761.50 | 1644.8 ± 489.96 | 2040.5 ± 757.57 | 214.5 ± 64.07 | 159.1 ± 56.83 | 250.6 ± 120.15 | 152.6 ± 41.05 | 283.8 ± 115.21 |
| Temperature | °C | 16.9 ± 5.40 | 16.8 ± 4.03 | 18.0 ± 4.14 | 16.6 ± 3.87 | 17.7 ± 4.20 | 17.7 ± 4.58 | 15.9 ± 4.18 | 17.3 ± 4.31 | 15.3 ± 4.23 | 17.0 ± 4.15 |
| Biochemical oxygen demand | mg/L | 34.7 ± 12.99 | 23.1 ± 9.35 | 12.1 ± 7.32 | 16.6 ± 7.07 | 8.3 ± 4.23 | 17.6 ± 8.00 | 13.4 ± 5.63 | 5.5 ± 6.00 | 6.7 ± 4.85 | 5.4 ± 3.95 |
| Chemical oxygen demand | mg/L | 129.2 ± 34.68 | 94.0 ± 31.13 | 90.7 ± 29.89 | 100.8 ± 27.65 | 103.1 ± 16.10 | 28.9 ± 14.47 | 31.3 ± 11.95 | 29.2 ± 10.71 | 17.2 ± 6.95 | 19.9 ± 7.28 |
| Ammonia–nitrogen | mg/L | 0.4 ± 0.19 | 0.5 ± 0.23 | 0.3 ± 0.14 | 0.3 ± 0.13 | 0.3 ± 0.11 | 0.2 ± 0.22 | 0.1 ± 0.07 | 0.1 ± 0.07 | 0.1 ± 0.04 | 0.1 ± 0.15 |
| Nitrate–nitrogen | mg/L | 8.9 ± 6.38 | 10.7 ± 7.92 | 16.3 ± 4.89 | 8.5 ± 8.42 | 15.0 ± 8.59 | 1.3 ± 1.21 | 1.3 ± 0.77 | 0.7 ± 0.77 | 1.0 ± 0.64 | 0.3 ± 0.28 |
| Ortho-phosphate–phosphorus | mg/L | 59.1 ± 14.16 | 48.0 ± 13.76 | 16.3 ± 3.00 | 43.0 ± 13.78 | 17.3 ± 5.63 | 8.4 ± 4.36 | 11.9 ± 6.36 | 3.0 ± 1.77 | 8.5 ± 4.03 | 3.7 ± 1.29 |
| Element [58,59] | |||||||||||
| Aluminium (Al) | mg/L | 2.13 ± 0.869 | 2.33 ± 1.321 | 1.56 ± 0.880 | 2.98 ± 1.218 | 3.61 ± 2.306 | 0.52 ± 0.528 | 0.12 ± 0.094 | 0.37 ± 0.232 | 0.36 ± 0.189 | 0.73 ± 0.420 |
| Boron (B) | mg/L | 0.57 ± 0.068 | 0.55 ± 0.211 | 0.44 ± 0.202 | 0.54 ± 0.160 | 0.39 ± 0.078 | 0.14 ± 0.067 | 0.13 ± 0.069 | 0.08 ± 0.005 | 0.12 ± 0.064 | 0.08 ± 0.006 |
| Calcium (Ca) | mg/L | 36.08 ± 8.750 | 42.49 ± 4.386 | 77.22 ± 42.765 | 37.39 ± 4.030 | 145.67 ± 92.506 | 10.54 ± 0.853 | 11.44 ± 0.944 | 60.11 ± 13.881 | 10.74 ± 0.739 | 65.46 ± 37.361 |
| Cadmium (Cd) | mg/L | 7.36 ± 2.981 | 5.82 ± 2.238 | 4.61 ± 2.126 | 6.40 ± 1.984 | 6.87 ± 2.628 | 0.09 ± 0.056 | 0.08 ± 0.097 | 0.02 ± 0.021 | 0.09 ± 0.083 | 0.05 ± 0.046 |
| Chromium (Cr) | mg/L | 3.20 ± 0.918 | 3.22 ± 1.736 | 2.86 ± 1.328 | 4.76 ± 1.215 | 4.75 ± 2.021 | 0.04 ± 0.063 | 0.05 ± 0.069 | 0.04 ± 0.031 | 0.07 ± 0.074 | 0.06 ± 0.054 |
| Copper (Cu) | mg/L | 1.44 ± 0.435 | 1.15 ± 0.385 | 0.98 ± 0.308 | 1.30 ± 0.301 | 1.47 ± 0.247 | 0.16 ± 0.058 | 0.07 ± 0.081 | 0.04 ± 0.032 | 0.10 ± 0.091 | 0.06 ± 0.057 |
| Iron (Fe) | mg/L | 6.41 ± 2.476 | 5.45 ± 1.657 | 5.03 ± 1.475 | 7.02 ± 1.801 | 8.69 ± 2.012 | 0.21 ± 0.102 | 0.14 ± 0.080 | 0.39 ± 0.218 | 0.20 ± 0.100 | 0.93 ± 0.759 |
| Potassium (K) | mg/L | 60.16 ± 1.684 | 44.90 ± 2.827 | 56.58 ± 19.919 | 45.77 ± 5.160 | 59.62 ± 20.132 | 4.04 ± 0.448 | 2.99 ± 0.216 | 17.59 ± 16.141 | 3.62 ± 0.438 | 20.16 ± 19.003 |
| Magnesium (Mg) | mg/L | 17.16 ± 2.119 | 17.77 ± 3.477 | 12.84 ± 6.124 | 16.24 ± 1.971 | 12.97 ± 3.785 | 1.45 ± 0.191 | 1.55 ± 0.195 | 0.84 ± 0.224 | 1.38 ± 0.161 | 0.78 ± 0.330 |
| Manganese (Mn) | mg/L | 0.98 ± 0.257 | 0.35 ± 0.249 | 0.46 ± 0.212 | 1.01 ± 0.223 | 0.86 ± 0.457 | 0.17 ± 0.084 | 0.05 ± 0.077 | 0.04 ± 0.033 | 0.06 ± 0.074 | 0.10 ± 0.094 |
| Sodium (Na) | mg/L | 62.68 ± 14.538 | 55.09 ± 11.391 | 55.85 ± 12.850 | 55.22 ± 11.852 | 55.59 ± 12.232 | 14.32 ± 1.662 | 13.91 ± 1.648 | 15.42 ± 3.280 | 13.15 ± 1.199 | 15.69 ± 5.272 |
| Nickel (Ni) | mg/L | 0.05 ± 0.065 | 0.10 ± 0.091 | 0.05 ± 0.077 | 0.09 ± 0.081 | 0.04 ± 0.033 | 0.04 ± 0.065 | 0.05 ± 0.081 | 0.00 ± 0.012 | 0.05 ± 0.080 | 0.01 ± 0.010 |
| Zinc (Zn) | mg/L | 4.25 ± 1.500 | 3.12 ± 0.872 | 2.78 ± 0.859 | 3.90 ± 0.972 | 4.32 ± 0.787 | 0.21 ± 0.159 | 0.11 ± 0.094 | 0.06 ± 0.050 | 0.13 ± 0.068 | 0.11 ± 0.089 |
| Parameter [57] | Unit | 2-day HRT (TW effluent) | 7-day HRT (TW effluent) | ||||||||
| C1 | C2 | C3 | C4 | ||||||||
| pH | – | 6.7 ± 0.39 | 7.4 ± 0.60 | 6.6 ± 0.39 | 7.1 ± 0.52 | ||||||
| Redox potential | mV | 42.2 ± 16.50 | 9.6 ± 28.10 | 44.1 ± 17.06 | 25.1 ± 24.68 | ||||||
| Turbidity | NTU | 9.3 ± 6.61 | 4.2 ± 4.37 | 12.7 ± 12.56 | 3.7 ± 3.47 | ||||||
| Total suspended solids | mg/L | 14.3 ± 8.16 | 3.9 ± 2.93 | 17.8 ± 13.69 | 4.3 ± 5.79 | ||||||
| Electronic conductivity | µS/cm | 84.4 ± 12.15 | 81.5 ± 9.94 | 92.9 ± 27.28 | 87.1 ± 20.83 | ||||||
| Dissolved oxygen | mg/L | 9.0 ± 0.87 | 10.4 ± 0.70 | 8.9 ± 1.09 | 10.8 ± 1.07 | ||||||
| Colour | Pa/Co | 44.3 ± 30.56 | 8.6 ± 7.66 | 56.1 ± 31.45 | 12.7 ± 9.73 | ||||||
| Temperature | °C | 16.5 ± 3.76 | 16.8 ± 4.04 | 15.1 ± 4.20 | 15.5 ± 4.17 | ||||||
| Biochemical oxygen demand | mg/L | 7.3 ± 3.45 | 5.4 ± 4.03 | 9.1 ± 5.05 | 6.7 ± 4.65 | ||||||
| Chemical oxygen demand | mg/L | 15.9 ± 7.74 | 6.3 ± 2.84 | 17.6 ± 6.74 | 7.0 ± 2.48 | ||||||
| Ammonia–nitrogen | mg/L | 0.1 ± 0.12 | 0.1 ± 0.14 | 0.1 ± 0.04 | 0.1 ± 0.05 | ||||||
| Nitrate–nitrogen | mg/L | 1.1 ± 0.75 | 0.8 ± 0.53 | 0.9 ± 0.42 | 0.8 ± 0.54 | ||||||
| Ortho-phosphate–phosphorus | mg/L | 2.8 ± 1.82 | 2.4 ± 0.63 | 3.4 ± 1.47 | 2.4 ± 0.86 | ||||||
| Element [58,59] | |||||||||||
| Aluminium (Al) | mg/L | 0.01 ± 0.006 | 0.01 ± 0.007 | 0.08 ± 0.092 | 0.09 ± 0.101 | ||||||
| Boron (B) | mg/L | 0.02 ± 0.018 | 0.03 ± 0.009 | 0.05 ± 0.061 | 0.05 ± 0.059 | ||||||
| Calcium (Ca) | mg/L | 9.96 ± 0.549 | 9.78 ± 0.552 | 9.67 ± 0.591 | 9.51 ± 0.476 | ||||||
| Cadmium (Cd) | mg/L | 0.01 ± 0.006 | 0.00 ± 0.006 | 0.04 ± 0.071 | 0.05 ± 0.071 | ||||||
| Chromium (Cr) | mg/L | 0.00 ± 0.005 | 0.00 ± 0.005 | 0.03 ± 0.063 | 0.03 ± 0.063 | ||||||
| Copper (Cu) | mg/L | 0.01 ± 0.006 | 0.01 ± 0.008 | 0.04 ± 0.073 | 0.05 ± 0.078 | ||||||
| Iron (Fe) | mg/L | 0.02 ± 0.007 | 0.02 ± 0.009 | 0.05 ± 0.069 | 0.05 ± 0.066 | ||||||
| Potassium (K) | mg/L | 0.35 ± 0.049 | 0.69 ± 0.261 | 0.50 ± 0.492 | 0.52 ± 0.127 | ||||||
| Magnesium (Mg) | mg/L | 1.10 ± 0.123 | 1.10 ± 0.138 | 1.20 ± 0.119 | 1.16 ± 0.120 | ||||||
| Manganese (Mn) | mg/L | 0.01 ± 0.010 | 0.00 ± 0.009 | 0.04 ± 0.070 | 0.04 ± 0.069 | ||||||
| Sodium (Na) | mg/L | 6.62 ± 0.721 | 6.69 ± 0.869 | 6.80 ± 0.085 | 6.35 ± 0.105 | ||||||
| Nickel (Ni) | mg/L | 0.01 ± 0.023 | 0.01 ± 0.023 | 0.04 ± 0.075 | 0.04 ± 0.075 | ||||||
| Sample | Aluminium | Boron | Calcium | Cadmium | Chromium | Copper | Iron | Potassium | Magnesium | Manganese | Sodium | Nickel | Zinc | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Al) | (B) | (Ca) | (Cd) | (Cr) | (Cu) | (Fe) | (K) | (Mg) | (Mn) | (Na) | (Ni) | (Zn) | ||
| Water (mg/L) | 5 | 3 | 20 | 0.01 | 0.1 | 0.2 | 5 | 2 | 5 | 0.2 | 40 | 0.2 | 2 | [1] |
| – | – | – | – | 0.55 | 0.017 | 0.5 | – | – | – | – | 1.4 | 0.2 | [2] | |
| 5-20 | – | – | 0.01–0.05 | 0.1–1 | 0.2–5 | 5–20 | – | – | 0.2–10 | – | 0.2–2 | 10 | [11] | |
| – | – | – | 0.01 | 0.1 | 0.2 | – | – | – | 0.2 | – | 0.2 | 2 | [71] | |
| Soil (mg/kg) | – | – | – | 3–6 | – | 270 | – | – | – | – | – | 150 | 600 | [29] |
| – | – | – | 3 | 150 | 140 | – | – | – | – | – | 75 | 300 | [31] | |
| – | – | – | – | 100 | 30 | – | – | – | – | – | 80 | 200 | [32] | |
| – | – | 3 | – | 100 | 100 | 50,000 | – | – | 2000 | – | 50 | 300 | [72] | |
| – | – | – | 100 | 100 | – | – | – | – | – | – | – | 1500 | [73] | |
| – | – | – | 100 | 20 | 0.3 | – | – | – | – | – | 140 | – | [74] | |
| Crops (mg/kg) | – | – | – | 0.1 | 2.3 | 73.3 | 425 | – | – | 500 | – | 67 | 100 | [27] |
| – | – | – | 0.05 | – | 20 | – | – | – | – | – | 50 | [31] | ||
| – | – | – | 0.02 | 1.3 | 10 | – | – | – | – | – | 10 | 0.6 | [32] |
| Parameter | Class A | Class B | Class C | Class D | Class E |
|---|---|---|---|---|---|
| Quality class | Outstanding | Very good | Good | Satisfactory | Unsatisfactory |
| Approximate Codex Standard | “Extra” Class | Class I | Class II | Not applicable | Not applicable |
| Length (L, mm) | Very long (L ≥ 80) | Long (80 > L ≥ 60) | Medium (60 > L ≥ 40) | Short (40 > L ≥ 20) | Very short (L < 20) |
| Width (W, mm) | Very wide (W ≥ 20) | Wide (20 > W ≥ 16) | Medium (16 > W ≥ 12) | Slim (12 > W ≥ 8) | Very slim (W < 8) |
| Fresh weight (w, gram) | Very large (w ≥ 9) | Large (9 > w ≥ 7) | Medium (7 > w ≥ 5) | Small (5 > w ≥ 3) | Very small (w < 3) |
| Bending (L/W) | Characteristically (L/W ≥ 3.5) | Characteristically (L/W ≥ 3.5) | Characteristically (L/W ≥ 3.5) | Uncharacteristically (L/W < 3.5) | Uncharacteristically (L/W < 3.5) |
| Price (Sterling, pence/g) | 2.00 | 1.00 | 0.50 | 0.25 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almuktar, S.A.A.A.N.; Abed, S.N.; Scholz, M.; Uzomah, V.C. Assessment of Capsicum annuum L. Grown in Controlled and Semi-Controlled Environments Irrigated with Greywater Treated by Floating Wetland Systems. Agronomy 2021, 11, 1817. https://doi.org/10.3390/agronomy11091817
Almuktar SAAAN, Abed SN, Scholz M, Uzomah VC. Assessment of Capsicum annuum L. Grown in Controlled and Semi-Controlled Environments Irrigated with Greywater Treated by Floating Wetland Systems. Agronomy. 2021; 11(9):1817. https://doi.org/10.3390/agronomy11091817
Chicago/Turabian StyleAlmuktar, Suhad A. A. A. N., Suhail N. Abed, Miklas Scholz, and Vincent C. Uzomah. 2021. "Assessment of Capsicum annuum L. Grown in Controlled and Semi-Controlled Environments Irrigated with Greywater Treated by Floating Wetland Systems" Agronomy 11, no. 9: 1817. https://doi.org/10.3390/agronomy11091817