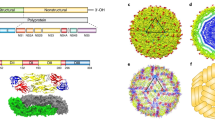
Abstract
Flaviviruses, the viruses in the Flavivirus genus, Flaviviridae family, cause diseases with varying degrees of severity in human beings, including fevers (Yellow fever, Dengue fever, West Nile fever, Zika fever, etc.) and encephalites (tick-borne encephalitis, Japanese encephalitis, Powassan encephalitis, etc.). Preventive vaccines have been produced against only some of these infectious agents, with their development being mainly hindered by the neurovirulence of flaviviruses, as well as by a long-known phenomenon, namely, the antibody-dependent enhancement (ADE) of infection characteristic of these viruses. The use of serum therapy in the case of flaviviruses has several limitations. Donor blood. which serves as a raw material to obtain specific immunoglobulins, is always a limited resource, and different blood samples are not standardized by the level of protective antibodies they contain. The risk of ADE is also a significant limitation. A number of technologies are currently available that allow searching for antibodies with desired biological characteristics, such as high efficiency and broad neutralization ability with no ADE induction. This paper discusses the monoclonal antibodies obtained using cell engineering techniques including hybridoma mouse, recombinant chimeric (humanized) mouse/human or primate/human, and recombinant wholly human antibodies, which are currently studied in vitro and in vivo as potential therapeutic agents inhibiting the entry of flaviviruses into sensitive host cells and/or restricting their replication.
Similar content being viewed by others
REFERENCES
L’vov, D.K., Alekseev, K.P., Alimbarova, L.M., Aliper, T.I., Al’khovskii, S.V., Andronova, V.L., et al., Rukovodstvo po virusologii (Virology Guide), Moscow: Meditsinskoe Informatsionnoe Agentstvo, 2013.
Grard, G., Moureau, G., Charrel, R.N., Holmes, E.C., Gould, E.A., and de Lamballerie, X., Genomics and evolution of Aedes-borne flaviviruses, J. Gen. Virol., 2010, vol. 91, part 1, pp. 87–94.
International Committee on Taxonomy of Viruses, ICTV. https://talk.ictvonline.org/taxonomy. Accessed May 5, 2020.
Tsai, W.Y., Lin, H.E., and Wang, W.K., Complexity of human antibody response to dengue virus: Implication for vaccine development, Front. Microbiol., 2017, vol. 8, p. 1372. https://doi.org/10.3389/fmicb.2017.01372
Musso, D. and Gubler, D.J., Zika virus, Clin. Microbiol. Rev., 2016, vol. 29, pp. 487–524. https://doi.org/10.1128/CMR.00072-15
Putintseva, E.V., Smelyanskii, V.P., Pak, V.A., Borodai, N.V., Zhukov, K.V., Manankov, V.V., et al., Epidemic Situation on West-Nile fever in 2014 in the territory of the Russian Federation and around the world, and prognosis for its development in 2015, Probl. Osobo Opasnykh Infekts., 2015, no. 1, pp. 29—36.
Putintseva, E.V., Smelyanskii, V.P., Borodai, N.V., Manankov, V.V., Tkachenko, G.A., Shpak, I.M., et al., West Nile fever across the world and on the territory of the Russian Federation in 2015, forecast of the epidemic situation development in 2016, Probl. Osobo Opasnykh Infekts., 2017, no. 1, pp. 29–36. https://doi.org/10.21055/0370-1069-2016-1-33-39
Putintseva, E.V., Smelyanskii, V.P., Borodai, N.V., Alekseichik, I.O., Shakhov, L.O., Tkachenko, G.A., et al., West Nile fever worldwide and in the territory of the Russian Federation in 2016, and forecast of epidemic situation development in 2017, Probl. Osobo Opasnykh Infekts., 2017, vol. 1, pp. 29–36. https://doi.org/10.21055/0370-1069-2017-1-29-36
Putintseva, E.V., Smelyanskii, V.P., Alekseychik, I.O., Borodai, N.V., Chesnokova, S.N., Alieva, A.K., et al., Results of monitoring over the West Nile fever pathogen in the territory of the Russian Federation in 2017. Forecast of epidemic situation development in Russia in 2018, Probl. Osobo Opasnykh Infekts., 2018, vol. 28, no. 1, pp. 56–62. https://doi.org/10.21055/0370-1069-2018-1-56-62
Alekseychik, I.O., Putintseva, E.V., Smelyanskii, V.P., Boroday, N.V., Alieva, A.K., Agarkova, E.A., et al., Peculiarities of the epidemic situation on West Nile fever in the territory of the Russian Federation in 2018 and forecast of its development in 2019, Probl. Partic. Dangerous Infect., 2019, no. 1, pp. 17–25. https://doi.org/10.21055/0370-1069-2019-1-17-25
Center for Disease Control and Prevention, CDC. https://www.cdc.gov/westnile/statsmaps/preliminarymapsdata2019/index.html. Accessed May 5, 2020.
Baikov, I.K., Development and research of recombinant antibodies against tick borne encephalitis virus, Cand. Sci. (Med.) Dissertation, Novosibirsk, 2015.
Li, Y., Hou, L., Ye, J., Liu, X., Dan, H., Jin, M., et al., Development of a convenient immunochromatographic strip for the diagnosis of infection with Japanese encephalitis virus in swine, J. Virol. Methods, 2010, vol. 168, nos. 1–2, pp. 51–56. https://doi.org/10.1016/j.jviromet.2010.04.015
Saifullin, M.A., Clinical and laboratory characteristic of imported cases of Dengue Hemorrhagic Fever, Cand. Sci. (Med.) Dissertation, Moscow, 2017. https://www.dissercat.com/content/kliniko-laboratornaya-kharakteristika-zavoznykh-sluchaev-likhoradki-denge. Accessed September 9, 2019.
Sikka, V., Chattu, V.K., Popli, R.K., Galwankar, S.C., Kelkar, D., Sawicki, S.G., et al., The emergence of zika virus as a global health security threat: A review and a consensus statement of the INDUSEM Joint working Group (JWG), J. Global Infect. Dis., 2016, vol. 8, no. 1, pp. 3–15. https://doi.org/10.4103/0974-777X.176140
Fox, J.P., Fonseca da Cunha, J., and Kossobudzki, S.L., Additional observations on the duration of humoral immunity following vaccination with the 17D strain of yellow fever virus, Am. J. Hyg., 1948, vol. 47, no. 1, pp. 64–70. https://doi.org/10.1093/oxfordjournals.aje.a119186
Durbin, A. and Wilder-Smith, A., An update on Zika vaccine developments, Expert Rev. Vaccines, 2017, vol. 16, no. 8, pp. 781–787. https://doi.org/10.1080/14760584.2017.1345309
Morozova, O.V., Isaeva, E.I., and Vyazov, S.O., New approaches to the treatment of flavivirus infections, Vopr. Virusol., 2015, vol. 60, no. 6, pp. 5–9. https://cyberleninka.ru/article/v/novye-podhody-k-lecheniyu-flavivirusnyh-infektsiy. Accessed September 9, 2019.
Priyamvada, L., Quicke, K.M., Hudson, W.H., Onlamoon, N., Sewatanon, J., Edupuganti, S., et al., Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus, Proc. Natl. Acad. Sci. U. S. A., 2016, vol. 113, no. 28, pp. 7852–7857. https://doi.org/10.1073/pnas.1607931113
Yu, L., Wang, R., Gao, F., Li, M., Liu, J., Wang, J., et al., Delineating antibody recognition against Zika virus during natural infection, JCI Insight, 2017, vol. 2, no. 12, pp. 1–16. https://doi.org/10.1172/jci.insight.93042
Köhler, G. and Milstein, C., Continuous cultures of fused cells secreting antibody of predefined specificity, Nature, 1975, vol. 256, no. 5517, pp. 495–497. https://doi.org/10.1038/256495a0
Deev, S.M. and Polyanovskii, O.L., Monoclonal antibodies for diagnosis and therapy, Biotekhnologiya, 2008, vol. 2, pp. 3–13.
Nikolenko, G.N., Development of recombinant antibodies against tick borne encephalitis virus and research of their properties, Cand. Sci. (Med.) Dissertation, Kol’tsovo, 1999. https://www.dissercat.com/content/sozdanie-rekombinantnykh-antitel-protiv-virusa-kleshchevogo-entsefalita-i-izuchenie-ikh-svoistv. Accessed September 9, 2019.
Pereboev, A., Borisevich, V., Tsuladze, G., Shakhmatov, M., Hudman, D., Kazachinskaia, E., et al., Genetically delivered antibody protects against West Nile virus, Antiviral Res., 2008, vol. 77, no. 1, pp. 6–13. https://doi.org/10.1016/j.antiviral.2007.08.010
Levanov, L.N., Matveev, L.E., Goncharova, E.P., Lebedev, L.R., Ryzhikov, A.B., Yun, T.E., et al., Chimeric antibodies against tick-borne encephalitis virus, Vaccine, 2010, vol. 28, no. 32, pp. 5265–5271. https://doi.org/10.1016/j.vaccine.2010.05.060
Cockburn, J.J.B., Navarro Sanchez, M.E., Fretes, N., Urvoas, A., Staropoli, I., Kikuti, C.M., et al., Mechanism of dengue virus broad cross-neutralization by a monoclonal antibody, Structure, 2012, vol. 20, no. 2, pp. 303–314. https://doi.org/10.1016/j.str.2012.01.001
Smith, S.A., de Alwis, A.R., Kose, N., Harris, E., Ibarra, K.D., Kahle, K.M., et al., The potent and broadly neutralizing human dengue virus-specific monoclonal antibody 1C19 reveals a unique cross-reactive epitope on the bc loop of domain II of the envelope protein, MBio, 2013, vol. 4, no. 6, p. e00873-13. https://doi.org/10.1128/mBio.00873-13
Lai, C.J., Goncalvez, A.P., Men, R., Wernly, C., Donau, O., Engle, R.E., et al., Epitope determinants of a chimpanzee dengue virus type 4 (DENV-4)-neutralizing antibody and protection against DENV-4 challenge in mice and rhesus monkeys by passively transferred humanized antibody, J. Virol., 2007, vol. 81, no. 23, pp. 12766–12774. https://doi.org/10.1128/JVI.01420-07
Throsby, M., Geuijen, C., Goudsmit, J., Bakker, A.Q., Korimbocus, J., Kramer, R.A., et al., Isolation and characterization of human monoclonal antibodies from individuals infected with West Nile virus, J. Virol., 2006, vol. 80, no. 14, pp. 6982–6992. https://doi.org/10.1128/JVI.00551-06
Hasan, S.S., Miller, A., Sapparapu, G., Fernandez, E., Klose, T., Long, F., et al., A human antibody against Zika virus crosslinks the E protein to prevent infection, Nat. Commun., 2017, vol. 8, no. 1, p. 14722. https://doi.org/10.1038/ncomms14722
Adungo, F., Yu, F., Kamau, D., Inoue, S., Hayasaka, D., Posadas-Herrera, G., et al., Development and characterization of monoclonal antibodies to yellow fever virus and application in antigen detection and IgM capture enzyme-linked immunosorbent assay, Clin. Vaccine Immunol., 2016, vol. 23, no. 8, pp. 689–697. https://doi.org/10.1128/CVI.00209-16
Davis, E.H. and Barrett, A.D.T., Structure-function of the yellow fever virus envelope protein: Analysis of antibody epitopes, Viral Immunol., 2020, vol. 33, no. 1, pp. 12–21. https://doi.org/10.1089/vim.2019.0107
Thibodeaux, B.A., Garbino, N.C., Liss, N.M., Piper, J., Schlesinger, J.J., Blair, C.D., et al., A humanized IgG but not IgM antibody is effective in prophylaxis and therapy of yellow fever infection in an AG129/17D-204 peripheral challenge mouse model, Antiviral Res., 2012, vol. 94, no. 1, pp. 1–8. https://doi.org/10.1016/j.antiviral.2012.02.001
The U.S. National Library of Medicine. https://clinicalttrials.gov/ct2/show/NCT03776786. Accessed May 5, 2020.
Tomashek, K.M., Challberg, M., Nayak, S.U., and Schiltz, H.F., Disease resurgence, production capability issues and safety concerns in the context of an aging population: Is there a need for a new yellow fever vaccine?, Vaccines, 2019, vol. 7, no. 4, p. 179. https://doi.org/10.3390/vaccines7040179
Crill, W.D. and Roehrig, J.T., Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells, J. Virol., 2001, vol. 75, no. 16, pp. 7769–7773. https://doi.org/10.1128/JVI.75.16.7769-7773.2001
Shrestha, B., Brien, J.D., Sukupolvi-Petty, S., Austin, S.K., Edeling, M.A., Kim, T., et al., The development of therapeutic antibodies that neutralize homologous and heterologous genotypes of dengue virus type 1, PLoS Pathog., 2010, vol. 6, no. 4, p. e1000823. https://doi.org/10.1371/journal.ppat.1000823
Sun, H., Chen, Q., and Lai, H., Development of antibody therapeutics against flaviviruses, Int. J. Mol. Sci., 2017, vol. 19, no. 1, p. 54. https://doi.org/10.3390/ijms19010054
Dai, L., Song, J., Lu, X., Deng, Y.Q., Musyoki, A.M., Cheng, H., et al., Structures of the Zika virus envelope protein and its complex with a flavivirus broadly protective antibody, Cell Host Microbe, 2016, vol. 19, no. 5, pp. 696–704. https://doi.org/10.1016/j.chom.2016.04.013
Wong, Y.H., Kumar, A., Liew, C.W., Tharakaraman, K., Srinivasaraghavan, K., Sasisekharan, R., et al., Molecular basis for dengue virus broad cross-neutralization by humanized monoclonal antibody, Sci. Rep., vol. 8, no. 1, pp. 1–17. https://doi.org/10.1038/s41598-018-26800-y
Goncalvez, A.P., Men, R., Wernly, C., Purcell, R.H., and Lai, C.J., Chimpanzee Fab fragments and a derived humanized immunoglobulin G1 antibody that efficiently cross-neutralize dengue type 1 and type 2 viruses, J. Virol., 2004, vol. 78, no. 23, pp. 12910–12918. https://doi.org/10.1128/JVI.78.23.12910-12918.2004
Dejnirattisai, W., Wongwiwat, W., Supasa, S., Zhang, X., Dai, X., Rouvinski, A., et al., A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus, Nat. Immunol., 2015, vol. 16, no. 2, pp. 170–177. https://doi.org/10.1038/ni.3058
Omi, N., Tokuda, Y., Ikeda, Y., Ueno, M., Mori, K., Sotozono, C., et al., Efficient and reliable establishment of lymphoblastoid cell lines by Epstein–Barr virus transformation from a limited amount of peripheral blood, Sci. Rep., 2017, vol. 7, no. 1, p. 43833. https://doi.org/10.1038/srep43833
Lok, S.M., Kostyuchenko, V., Nybakken, G.E., Holdaway, H.A., Battisti, A.J., and Sukupolvi-Petty, S., Binding of a neutralizing antibody to dengue virus alters the arrangement of surface glycoproteins, Nat. Struct. Mol. Biol., 2008, vol. 15, no. 3, pp. 312–317. https://doi.org/10.1038/nsmb.1382
Fibriansah, G., Ibarra, K.D., Ng, T.S., Smith, S.A., Tan, J.L., Lim, X.N., et al., Cryo-EM structure of an antibody that neutralizes dengue virus type 2 by locking E protein dimers, Science, 2015, vol. 349, no. 6243, pp. 88–91. https://doi.org/10.1126/science.aaa8651
Teoh, E.P., Kukkaro, P., Teo, E.W., Lim, A.P., Tan, T.T., Yip, A., et al., The structural basis for serotype-specific neutralization of dengue virus by a human antibody, Sci. Transl. Med., 2012, vol. 4, no. 139, p. 139ra83. https://doi.org/10.1126/scitranslmed.3003888
Li, P.C., Liao, M.Y., Cheng, P.C., Liang, J.J., Liu, I.J., Chiu, C.Y., et al., Development of a humanized antibody with high therapeutic potential against dengue virus type 2, PLoS Neglected Trop. Dis., 2012, vol. 6, no. 5, p. e1636. https://doi.org/10.1371/journal.pntd.0001636
Shi, X., Deng, Y., Wang, H., Ji, G., Tan, W., Jiang, T., et al., A bispecific antibody effectively neutralizes all four serotypes of dengue virus by simultaneous blocking virus attachment and fusion, MAbs, 2016, vol. 8, no. 3, pp. 574–584. https://doi.org/10.1080/19420862.2016.1148850
The U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT04273217?term=antibody& cond=Dengue&draw=6&rank=48. Accessed May 5, 2020.
Gore, M.M., Gupta, A.K., Ayachit, V.M., Athawale, S.S., Ghosh, S.N., Banerjee, K., et al., Selection of a neutralization-escape variant strain of Japanese encephalitis virus using monoclonal antibody, Indian J. Med. Res., 1990, vol. 91, pp. 231–233. https://www.ncbi. nlm.nih.gov/pubmed/1697849. Accessed May 5, 2020.
Goncalvez, A.P., Chien, C.H., Tubthong, K., Gorshkova, I., Roll, C., Donau, O., et al., Humanized monoclonal antibodies derived from chimpanzee Fabs protect against Japanese encephalitis virus in vitro and in vivo, J. Virol., 2008, vol. 82, no. 14, pp. 7009–7021. https://doi.org/10.1128/JVI.00291-08
Razumov, I.A., Kazachinskaia, E.I., Ternovoi, V.A., Protopopova, E.V., Galkina, I.V., Gromashevskii, V.L., et al., Neutralizing monoclonal antibodies against Russian strain of the West Nile virus, Viral Immunol., 2005, vol. 18, no. 3, pp. 558–568. https://doi.org/10.1089/vim.2005.18.558
Oliphant, T., Engle, M., Nybakken, G.E., Doane, C., Johnson, S., Huang, L., et al., Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus, Nat. Med., 2005, vol. 11, no. 5, pp. 522–530. https://doi.org/10.1038/nm1240
Sun, H., Chen, Q., and Lai, H., Development of antibody therapeutics against flaviviruses, Int. J. Mol. Sci., 2018, vol. 19, no. 1, p. 54. https://doi.org/10.3390/ijms19010054
Nybakken, G.E., Oliphant, T., Johnson, S., Burke, S., Diamond, M.S., Fremont, D.H., et al., Structural basis of West Nile virus neutralization by a therapeutic antibody, Nature, 2005, vol. 437, no. 7059, pp. 764–769. https://doi.org/10.1038/nature03956
Oliphant, T., Nybakken, G.E., Austin, S.K., Xu, Q., Bramson, J., Loeb, M., et al., Induction of epitope-specific neutralizing antibodies against West Nile virus, J. Virol., 2007, vol. 81, no. 21, pp. 11828–11839. https://doi.org/10.1128/jvi.00643-07
Dowd, K.A., Jost, C.A., Durbin, A.P., Whitehead, S.S., and Pierson, T.C., A dynamic landscape for antibody binding modulates antibody-mediated neutralization of West Nile virus, PLoS Pathog., 2011, vol. 7, no. 6, p. e1002111. https://doi.org/10.1371/journal.ppat.1002111
Beigel, J.H., Nordstrom, J.L., Pillemer, S.R., Roncal, C., Goldwater, D.R., Li, H., et al., Safety and pharmacokinetics of single intravenous dose of MGAWN1, a novel monoclonal antibody to West Nile virus, Antimicrob. Agents Chemother., 2010, vol. 54, no. 6, pp. 2431–2436. https://doi.org/10.1128/AAC.01178-09
The U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT00515385?term=antibody& cond=West+Nile+Virus&draw=2&rank=3. Accessed May 5, 2020.
The U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT00927953?term=antibody &cond=West+Nile+Virus&draw=2&rank=9. Accessed May 5, 2020.
Vogt, M.R., Moesker, B., Goudsmit, J., Jongeneelen, M., Austin, S.K., Oliphant, T., et al., Human monoclonal antibodies against West Nile virus induced by natural infection neutralize at a postattachment step, J. Virol., 2009, vol. 83, no. 13, pp. 6494–6507. https://doi.org/10.1128/JVI.00286-09
Volk, D.E., Beasley, D.W.C., Kallick, D.A., Holbrook, M.R., Barrett, A.D.T., Gorenstein, D.G., et al., Solution structure and antibody binding studies of the envelope protein domain III from the New York strain of West Nile virus, J. Biol. Chem., 2004, vol. 279, no. 37, pp. 38755–38761. https://doi.org/10.1074/jbc.M402385200
Goo, L., Debbink, K., Kose, N., Sapparapu, G., Doyle, M.P., Wessel, A.W., et al., A potently neutralizing human monoclonal antibody targeting an epitope in the West Nile virus E protein preferentially recognizes mature virions, Nat. Microbiol., 2019, vol. 4, no. 1, pp. 71–77. https://doi.org/10.1038/s41564-018-0283-7
Sánchez, M.D., Pierson, T.C., McAllister, D., Hanna, S.L., Puffer, B.A., Valentine, L.E., et al., Characterization of neutralizing antibodies to West Nile virus, Virology, 2005, vol. 336, no. 1, pp. 70–82. https://doi.org/10.1016/j.virol.2005.02.020
Sun, E.C., Ma, J.N., Liu, N.H., Yang, T., Zhao, J., Geng, H.W., et al., Identification of two linear B-cell epitopes from West Nile virus NS1 by screening a phage-displayed random peptide library, BMC Microbiol., 2011, vol. 11, no. 1, p. 160. https://doi.org/10.1186/1471-2180-11-160
Chung, K.M., Nybakken, G.E., Thompson, B.S., Engle, M.J., Marri, A., Fremont, D.H., et al., Antibodies against West Nile virus nonstructural protein NS1 prevent lethal infection through Fc γ receptor-dependent and -independent mechanisms, J. Virol., 2006, vol. 80, no. 3, pp. 1340–1351. https://doi.org/10.1128/jvi.80.3.1340-1351.2006
Zhao, H., Fernandez, E., Dowd, K.A., Speer, S.D., Platt, D.J., Gorman, M.J., et al., Structural basis of Zika virus-specific antibody protection, Cell, 2016, vol. 166, no. 4, pp. 1016–1027. https://doi.org/10.1016/J.CELL.2016.07.020
Wang, S., Hong, S., Deng, Y.Q., Ye, Q., Zhao, L.Z., Zhang, F.C., et al., Transfer of convalescent serum to pregnant mice prevents Zika virus infection and microcephaly in offspring, Cell Res., 2017, vol. 27, no. 1, pp. 158–160. https://doi.org/10.1038/cr.2016.144
Wang, Q., Yang, H., Liu, X., Dai, L., Ma, T., Qi, J., et al., Molecular determinants of human neutralizing antibodies isolated from a patient infected with Zika virus, Sci. Transl. Med., 2016, vol. 8, no. 369, p. 369ra179. https://doi.org/10.1126/scitranslmed.aai8336
Robbiani, D.F., Bozzacco, L., Keeffe, J.R., Khouri, R., Olsen, P.C., Gazumyan, A., et al., Recurrent potent human neutralizing antibodies to Zika virus in Brazil and Mexico, Cell, 2017, vol. 169, no. 4, pp. 597–609.e11. https://doi.org/10.1016/j.cell.2017.04.024
Wu, Y., Li, S., Du, L., Wang, C., Zou, P., Hong, B., et al., Neutralization of Zika virus by germline-like human monoclonal antibodies targeting cryptic epitopes on envelope domain III, Emerging Microbes Infect., 2017, vol. 6, no. 1, pp. 1–11. https://doi.org/10.1038/emi.2017.79
Wang, J., Bardelli, M., Espinosa, D.A., Pedotti, M., Ng, T.S., Bianchi, S., et al., A human Bi-specific antibody against Zika virus with high therapeutic potential, Cell, 2017, vol. 171, no. 1, pp. 229–241.e15. https://doi.org/10.1016/j.cell.2017.09.002
The U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT03443830. Accessed May 5, 2020.
Jiang, S. and Du, L., Advances in the research and development of therapeutic antibodies against the Zika virus, Cell. Mol. Immunol., 2019, vol. 16, no. 1, pp. 107–108. https://doi.org/10.1038/s41423-018-0043-x
Bailey, M.J., Duehr, J., Dulin, H., Broecker, F., Brown, J.A., Arumemi, F.O., et al., Human antibodies targeting Zika virus NS1 provide protection against disease in a mouse model, Nat. Commun., 2018, vol. 9, no. 1, p. 4560. https://doi.org/10.1038/s41467-018-07008-0
Li, X.Q., Chen, J., Huang, Y.F., Ding, X.X., Liu, L.D., Qiu, L.W., et al., Evaluation and analysis of dengue virus enhancing and neutralizing activities using simple high-throughput assays, Appl. Microbiol. Biotechnol., 2013, vol. 97, no. 14, pp. 6503–6511. https://doi.org/10.1007/s00253-013-5021-8
Oliphant, T., Engle, M., Nybakken, G.E., Doane, C., Johnson, S., Huang, L.S., et al., Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus, Nat. Med., 2005, vol. 11, no. 5, pp. 522–530. https://doi.org/10.1038/nm1240
Fernandez, E., Dejnirattisai, W., Cao, B., Scheaffer, S.M., Supasa, P., Wongwiwat, W., et al., Human antibodies to the dengue virus E-dimer epitope have therapeutic activity against Zika virus infection, Nat. Immunol., 2017, vol. 18, no. 11, pp. 1261–1269. https://doi.org/10.1038/ni.3849
The International Immunogenetics Information System. https://www.imgt.org/mAb-DB/mAbcard?AbId=436. Accessed May 5, 2020.
Funding
This work was supported by a state order to the Vector State Research Center of Virology and Biotechnology, Russian Federal State Agency for Health and Consumer Rights Surveillance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. No experimentation involving animals or human beings was performed by any of the authors.
Additional information
Translated by E. Martynova
ADDITIONAL INFORMATION
Nesmeyanova V.S.: https://orcid.org/0000-0003-1091-3586; e-mail: nesmeyanova_vs@vector.nsc.ru
Shcherbakov D.N.: https://orcid.org/0000-0001-8023-4453; e-mail: dnshcherbakov@gmail.com
Kazachinskaya E.I.: https://orcid.org/0000-0002-1856-6147; e-mail: alenakaz@vector.nsc.ru
About this article
Cite this article
Nesmeianova, V.S., Sherbakov, D.N. & Kazachinskaia, E.I. Using Flavivirus-Specific Monoclonal Antibodies to Study the Antigenic Structure of Flaviviruses and Develop Anti-Flavivirus Drugs. Mol. Genet. Microbiol. Virol. 36, 57–67 (2021). https://doi.org/10.3103/S0891416821020051
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0891416821020051