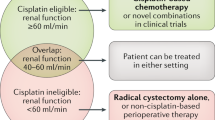
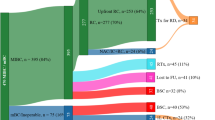
Abstract
The success of the use of novel therapies in the treatment of advanced urothelial carcinoma has contributed to growing interest in evaluating these therapies at earlier stages of the disease. However, trials evaluating these therapies in the neoadjuvant setting must have clearly defined study elements and appropriately selected end points to ensure the applicability of the trial and enable interpretation of the study results. To advance the development of rational trial design, a public workshop jointly sponsored by the US Food and Drug Administration and the Bladder Cancer Advocacy Network convened in August 2019. Clinicians, clinical trialists, radiologists, biostatisticians, patients, advocates and other stakeholders discussed key elements and end points when designing trials of neoadjuvant therapy for muscle-invasive bladder cancer (MIBC), identifying opportunities to refine eligibility, design and end points for neoadjuvant trials in MIBC. Although pathological complete response (pCR) is already being used as a co-primary end point, both individual-level and trial-level surrogacy for time-to-event end points, such as event-free survival or overall survival, remain incompletely characterized in MIBC. Additionally, use of pCR is limited by heterogeneity in pathological evaluation and the fact that the magnitude of pCR improvement that might translate into a meaningful clinical benefit remains unclear. Given existing knowledge gaps, capture of highly granular patient-related, tumour-related and treatment-related characteristics in the current generation of neoadjuvant MIBC trials will be critical to informing the design of future trials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Griffiths, G., Hall, R., Sylvester, R., Raghavan, D. & Parmar, M. K. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J. Clin. Oncol. 29, 2171–2177 (2011).
Grossman, H. B. et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder. Cancer 349, 859–866 (2003). This was the first randomized trial in MIBC that included pCR as a key end point, reported pCR results in neoadjuvant chemotherapy and control arms, and also survival results according to pCR status.
Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur. Urol. 48, 202–205; discussion 205–206 (2005).
Thompson, R. H. et al. Eligibility for neoadjuvant/adjuvant cisplatin-based chemotherapy among radical cystectomy patients. BJU Int. 113, E17–E21 (2014).
Jiang, D. M. et al. Defining cisplatin eligibility in patients with muscle-invasive bladder cancer. Nat. Rev. Urol. 18, 104–114 (2021).
Powles, T. et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N. Engl. J. Med. 384, 1125–1135 (2021).
Loriot, Y. et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 381, 338–348 (2019).
Dietrich, B., Siefker-Radtke, A. O., Srinivas, S. & Yu, E. Y. Systemic therapy for advanced urothelial carcinoma: current standards and treatment considerations. Am. Soc. Clin. Oncol. Educ. Book 38, 342–353 (2018).
Donat, S. M. et al. Potential impact of postoperative early complications on the timing of adjuvant chemotherapy in patients undergoing radical cystectomy: a high-volume tertiary cancer center experience. Eur. Urol. 55, 177–185 (2009).
Fisher, B., Gunduz, N. & Saffer, E. A. Influence of the interval between primary tumor removal and chemotherapy on kinetics and growth of metastases. Cancer Res. 43, 1488–1492 (1983).
Petrelli, F. et al. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: a meta-analysis. Eur. Urol. 65, 350–357 (2014). This meta-analysis of 13 trials demonstrates a correlation between pCR after neoadjuvant chemotherapy and improved long-term outcomes in MIBC.
FDA. Surrogate endpoint resources for drug and biologic development. Surrogate Endpoint Resources for Drug and Biologic Development. FDA https://www.fda.gov/drugs/development-resources/surrogate-endpoint-resources-drug-and-biologic-development (2018).
Korn, E. L., Sachs, M. C. & McShane, L. M. Statistical controversies in clinical research: assessing pathologic complete response as a trial-level surrogate end point for early-stage breast cancer. Ann. Oncol. 27, 10–15 (2016). The authors explain the difference between a trial-level surrogate and individual-level surrogate and reanalyse previous meta-analyses evaluating pCR as a trial-level surrogate for EFS and OS in breast cancer, finding no evidence that pCR is a trial-level surrogate in breast cancer or that it should be used to discourage further drug development of a new agent based on negative results for pCR.
Korn, E. L. & Freidlin, B. Surrogate and intermediate endpoints in randomized trials: what’s the goal? Clin. Cancer Res. 24, 2239–2240 (2018).
Prentice, R. L. Surrogate endpoints in clinical trials: definition and operational criteria. Stat. Med. 8, 431–440 (1989).
De Gruttola, V. G. et al. Considerations in the evaluation of surrogate endpoints in clinical trials. summary of a National Institutes of Health workshop. Control. Clin. Trials 22, 485–502 (2001).
Teramukai, S., Nishiyama, H., Matsui, Y., Ogawa, O. & Fukushima, M. Evaluation for surrogacy of end points by using data from observational studies: tumor downstaging for evaluating neoadjuvant chemotherapy in invasive bladder cancer. Clin. Cancer Res. 12, 139–143 (2006).
Molenberghs, G. et al. Statistical challenges in the evaluation of surrogate endpoints in randomized trials. Control. Clin. Trials 23, 607–625 (2002).
Burzykowski, T., Molenberghs, G. & Buyse, M. The validation of surrogate end points by using data from randomized clinical trials: a case-study in advanced colorectal cancer. J. R. Stat. Soc. Ser. A 167, 103–124 (2004). The authors describe a method of validating a binary end point as a surrogate end point for a time-to-event true end point, which has subsequently been used in several pooled analyses, including in Cortazar et al.22, which evaluated the relationship between pCR and EFS/OS in breast cancer.
Fleming, T. R. & Powers, J. H. Biomarkers and surrogate endpoints in clinical trials. Stat. Med. 31, 2973–2984 (2012).
Broglio, K. R. et al. Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes: a meta-analysis. JAMA Oncol. 2, 751–760 (2016).
Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384, 164–172 (2014).
Minckwitz, G. V. et al. Correlation of various pathologic complete response (pCR) definitions with long-term outcome and the prognostic value of pCR in various breast cancer subtypes: results from the German neoadjuvant meta-analysis. J. Clin. Oncol. 29, 1028–1028 (2011).
Spring, L. M. et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clin. Cancer Res. 26, 2838–2848 (2020).
Mieog, J. S., van der Hage, J. A. & van de Velde, C. J. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst. Rev. 2007, CD005002 (2007).
FDA. BLA 125409Orig1s051 Summary Review. Drugs@FDA https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/125409Orig1s051SumR.pdf (2013).
Baselga, J. et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N. Engl. J. Med. 366, 109–119 (2012).
von Minckwitz, G. et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N. Engl. J. Med. 377, 122–131 (2017).
FDA. BLA 125409Orig1s113s118 letter. Supplement approval fulfillment of postmarketing requirement and commitment. FDA https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/125409Orig1s113s118ltr.pdf (2017).
Schmid, P. et al. Pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 382, 810–821 (2020).
FDA. Combined FDA and Merck Sharp & Dohme Briefing Document for the February 9, 2021 Meeting of the Oncologic Drugs Advisory Committee. FDA https://www.fda.gov/media/145654/download (2021).
FDA. Oncologic Drugs Advisory Committee (ODAC) Meeting: Tuesday, February 9, 2021 (transcript). FDA https://fda.report/media/149022/ODAC-20210209-Transcript_0.pdf (2021).
Prowell, T. M., Beaver, J. A. & Pazdur, R. Residual disease after neoadjuvant therapy — developing drugs for high-risk early breast cancer. N. Engl. J. Med. 380, 612–615 (2019). This FDA’s perspective article summarizes the history of pCR in breast cancer trials and highlights the advantages of an approach enrolling patients without pCR in trials evaluating adjuvant therapy.
FDA. BLA 125409Orig1s051 cross discipline team leader review. FDA https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/125409Orig1s051CrossR.pdf (2013).
Berry, D. A. & Hudis, C. A. Neoadjuvant therapy in breast cancer as a basis for drug approval. JAMA Oncol. 1, 875–876 (2015).
Rosenblatt, R. et al. Pathologic downstaging is a surrogate marker for efficacy and increased survival following neoadjuvant chemotherapy and radical cystectomy for muscle-invasive urothelial bladder cancer. Eur. Urol. 61, 1229–1238 (2012).
No authors listed. Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial. Lancet 354, 533–540 (1999).
Apolo, A. B. et al. Eligibility and radiologic assessment in adjuvant clinical trials in bladder cancer. JAMA Oncol. 5, 1790–1798 (2019). The leaders of an FDA and National Cancer Institute workshop in 2017 summarized the workshop discussion in this paper, identifying areas where a uniform approach to eligibility and radiological assessment in adjuvant clinical trials in bladder was needed.
Galsky, M. D. et al. Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J. Clin. Oncol. 29, 2432–2438 (2011).
Ge, P. et al. Oncological outcome of primary and secondary muscle-invasive bladder cancer: a systematic review and meta-analysis. Sci. Rep. 8, 7543 (2018).
Pietzak, E. J. et al. Genomic differences between “primary” and “secondary” muscle-invasive bladder cancer as a basis for disparate outcomes to cisplatin-based neoadjuvant chemotherapy. Eur. Urol. 75, 231–239 (2019).
Chang, S. S. et al. Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO Guideline. J. Urol. 198, 552–559 (2017).
NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Bladder Cancer. NCCN https://www.nccn.org/professionals/physician_gls/PDF/bladder.pdf (2021).
Kim, L. H. C. & Patel, M. I. Transurethral resection of bladder tumour (TURBT). Transl. Androl. Urol. 9, 3056–3072 (2020).
Shinagare, A. B. et al. Metastatic pattern of bladder cancer: correlation with the characteristics of the primary tumor. AJR Am. J. Radiol. 196, 117–122 (2011).
Metwally, M. I. et al. The validity, reliability, and reviewer acceptance of VI-RADS in assessing muscle invasion by bladder cancer: a multicenter prospective study. Eur. Radiol. 31, 6949–6961 (2021).
Panebianco, V. et al. Multiparametric magnetic resonance imaging for bladder cancer: development of VI-RADS (Vesical Imaging-Reporting And Data System). Eur. Urol. 74, 294–306 (2018).
Witjes, J. A. et al. EAU-ESTRO-SIOG guidelines on muscle-invasive and metastatic bladder cancer. Eur. Urol. 64, 778–792 (2014).
van der Pol C. B. et al. ACR Appropriateness Criteria: pretreatment staging of muscle-invasive bladder cancer. ACR https://acsearch.acr.org/docs/69370/Narrative/ (2017).
Einerhand, S. M. H. et al. 18F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in muscle-invasive bladder cancer. Curr. Opin. Urol. 30, 654–664 (2020).
Goodfellow, H. et al. Role of fluorodeoxyglucose positron emission tomography (FDG PET)-computed tomography (CT) in the staging of bladder cancer. BJU Int. 114, 389–395 (2014).
Apolo, A. B. et al. Clinical value of fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in bladder cancer. J. Clin. Oncol. 28, 3973–3978 (2010).
Dason, S. et al. Utility of routine preoperative 18f-fluorodeoxyglucose positron emission tomography-computerized tomography in identifying pathological lymph node metastases at radical cystectomy. J. Urol. 204, 254–259 (2020).
Al-Daghmin, A. et al. External validation of preoperative and postoperative nomograms for prediction of cancer-specific survival, overall survival and recurrence after robot-assisted radical cystectomy for urothelial carcinoma of the bladder. BJU Int. 114, 253–260 (2014).
Galsky, M. D. et al. Web-based tool to facilitate shared decision making with regard to neoadjuvant chemotherapy use in muscle-invasive bladder cancer. JCO Clin. Cancer Inform. 1, 1–12 (2017).
Cahn, D. et al. MP58-12 clinical destiny of indeterminate pulmonary nodules in patients undergoing radical cystectomy for urothelial carcinoma of the bladder. J. Urol. 197, e776–e777 (2017).
Galsky, M. D. et al. Comparative effectiveness of treatment strategies for bladder cancer with clinical evidence of regional lymph node involvement. J. Clin. Oncol. 34, 2627–2635 (2016).
Galsky, M. D. et al. Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer 121, 2586–2593 (2015).
Peyton, C. C. et al. Downstaging and survival outcomes associated with neoadjuvant chemotherapy regimens among patients treated with cystectomy for muscle-invasive bladder cancer. JAMA Oncol. 4, 1535–1542 (2018).
Flaig, T. W. et al. SWOG S1314: a randomized phase II study of co-expression extrapolation (COXEN) with neoadjuvant chemotherapy for localized, muscle-invasive bladder cancer. J. Clin. Oncol. 37, 4506 (2019).
Koshkin, V. S. et al. Feasibility of cisplatin-based neoadjuvant chemotherapy in muscle-invasive bladder cancer patients with diminished renal function. Clin. Genitourin. Cancer 16, e879–e892 (2018).
Dash, A. et al. A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder. Cancer 113, 2471–2477 (2008).
Osterman, C. K. et al. Efficacy of split schedule versus conventional schedule neoadjuvant cisplatin-based chemotherapy for muscle-invasive bladder cancer. Oncologist 24, 688–690 (2019).
Powles, T. et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat. Med. 25, 1706–1714 (2019).
Necchi, A. et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, phase II study. J. Clin. Oncol. 36, 3353–3360 (2018).
Bajorin, D. F. et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N. Engl. J. Med. 384, 2102–2114 (2021).
Becker, R. E. N. et al. Clinical restaging and tumor sequencing are inaccurate indicators of response to neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur. Urol. 79, 364–371 (2021).
Herr, H., Lee, C., Chang, S. & Lerner, S. Standardization of radical cystectomy and pelvic lymph node dissection for bladder cancer: a collaborative group report. J. Urol. 171, 1823–1828 (2004).
Herr, H. W. Outcome of patients who refuse cystectomy after receiving neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur. Urol. 54, 126–132 (2008).
Robins, D. et al. Outcomes following clinical complete response to neoadjuvant chemotherapy for muscle-invasive urothelial carcinoma of the bladder in patients refusing radical cystectomy. Urology 111, 116–121 (2018).
Sternberg, C. N. et al. Can patient selection for bladder preservation be based on response to chemotherapy? Cancer 97, 1644–1652 (2003).
van der Pol, C. B. et al. Update on multiparametric MRI of urinary bladder cancer. J. Magn. Reson. Imaging 48, 882–896 (2018).
Necchi, A. et al. Multiparametric magnetic resonance imaging as a noninvasive assessment of tumor response to neoadjuvant pembrolizumab in muscle-invasive bladder cancer: preliminary findings from the PURE-01 study. Eur. Urol. 77, 636–643 (2020).
Choueiri, T. K. et al. Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: pathologic, radiologic, and biomarker correlates. J. Clin. Oncol. 32, 1889–1894 (2014).
Choi, S. J. et al. Urothelial phase CT for assessment of pathologic complete response after neoadjuvant chemotherapy in muscle-invasive bladder cancer. Eur. J. Radiol. 126, 108902 (2020).
Metser, U. et al. Detection of urothelial tumors: comparison of urothelial phase with excretory phase CT urography — a prospective study. Radiology 264, 110–118 (2012).
Zheng, J. et al. Development of a noninvasive tool to preoperatively evaluate the muscular invasiveness of bladder cancer using a radiomics approach. Cancer 125, 4388–4398 (2019).
Avulova, S. & Chang, S. S. Role and indications of organ-sparing “radical” cystectomy: the importance of careful patient selection and counseling. Urol. Clin. North Am. 45, 199–214 (2018).
Chang, S. S., Cole, E., Cookson, M. S., Peterson, M. & Smith, J. A. Jr. Preservation of the anterior vaginal wall during female radical cystectomy with orthotopic urinary diversion: technique and results. J. Urol. 168, 1442–1445 (2002).
Gschwend, J. E. et al. Extended versus limited lymph node dissection in bladder cancer patients undergoing radical cystectomy: survival results from a prospective, randomized trial. Eur. Urol. 75, 604–611 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01224665 (2021).
Sonpavde, G. et al. Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer 115, 4104–4109 (2009).
Splinter, T. A. et al. The prognostic value of the pathological response to combination chemotherapy before cystectomy in patients with invasive bladder cancer. European Organization for Research on Treatment of Cancer–Genitourinary Group. J. Urol. 147, 606–608 (1992).
Dash, A. et al. A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder: a retrospective experience. Cancer 113, 2471–2477 (2008).
Gress D. M. Principles of cancer staging. In AJCC Cancer Staging Manual 8th Edn (eds Amin, M. B. et al.) (Springer, 2021).
Martini, A. et al. Tumor downstaging as an intermediate endpoint to assess the activity of neoadjuvant systemic therapy in patients with muscle-invasive bladder cancer. Cancer 125, 3155–3163 (2019).
FDA. Pathologic complete response in neoadjuvant treatment of high-risk early-stage breast cancer: use as an endpoint to support accelerated approval. Guidance document: pathologic complete response in neoadjuvant treatment of high-risk early-stage breast cancer: use as an endpoint to support accelerated approval. FDA https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pathologic-complete-response-neoadjuvant-treatment-high-risk-early-stage-breast-cancer-use-endpoint (2020). This guidance is intended to assist investigators in designing trials with the use of pCR as an early end point to support accelerated approval, and discusses accepted definitions of pCR in breast cancer, describes trial designs and patient populations in which pCR may be accepted as reasonably likely to predict clinical benefit, and provides guidance on potential trial designs to verify clinical benefit for traditional approval.
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03661320 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03732677 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03924856 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03924895 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04209114 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04700124 (2021).
Acknowledgements
The authors thank F. Cross and D. Spillman for project management assistance; D. Zipursky Quale, L. Amiri-Kordestani and A. Ibrahim for support.
Author information
Authors and Affiliations
Contributions
E.C., A.B.A., V.D., A.M.K., S.P.L., E.P., E.Y.Y, C.W. and M.D.G. researched data for article. E.C., A.B.A., R.B., S.C., V.D., J.A.E., D.E.H., A.M.K., P.G.K., S.P.L., E.P., T.P., H.S., D.S., E.Y.Y., H.Z., J.A.B., R.P., C.W. and M.D.G. made substantial contributions to discussions of content. E.C., A.B.A., V.D., D.E.H., A.M.K., S.P.L., E.Y.Y., J.A.B., C.W. and M.D.G. wrote the article. E.C., A.B.A., V.D., K.B.G., P.G.K., E.P., J.A.B., C.W. and M.D.G. reviewed and edited the manuscript before submission
Corresponding author
Ethics declarations
Competing interests
E.C., A.B.A., K.B.G., P.G.K., T.P., H.S., D.S., H.Z., J.A.B., R.P. and C.W. are employees of the US government. R.B. receives honoraria from SWOG, the National Cancer Institute, the NCRA Working Group on Clinical Trials Enrollment and Retention, the Patient Advocate Steering Committee, the Cancer Care Delivery Research Steering Committee; he serves on committees with the National Comprehensive Cancer Network, the Bladder Cancer Advocacy Network, Stand Up to Cancer, Bladder Cancer (Editorial Board). V.D. receives funding from Samsung Healthcare; he serves on the advisory board for Deeptek and is a consultant for Radmetrix. J.A.E. has served as a consultant for Blue Earth Diagnostics, Boston Scientific, AstraZeneca, Taris Biomedical; and on advisory boards for Merck, Roivant Pharma, Myovant Sciences, Janssen, Bayer Healthcare, Progenics, Genentech and EMD Serono. A.M.K. receives funding from Adolor, Bristol-Myers Squibb (BMS), FKD Industries, FerGene, Heat Biologics, Merck, Photocure, SWOG, NIH/GU SPORE, AIBCCR, holds a patent for Cytokine Predictors of Response to Intravesical Therapy; he serves/served as a consultant or on the advisory board for Abbott Molecular, Arquer Diagnostics, ArTara, Asieris, AstraZeneca, BioClin Therapeutics, Biological Dynamics, BMS, Cepheid, CG Oncology, Cold Genesys, H3 Biomedicine/Eisai, Engene, FerGene, Ferring, Imagin Medical, Janssen, MdDxHealth, Medac, Merck, Pfizer, Photocure, ProTara, Roviant, Seattle Genetics, Sessen Bio, Theralase, TMC Innovation, US Biotest, and is a board member of the International Bladder Cancer Group. S.P.L. receives honoraria from UroToday, holds a patent for a TCGA classifier, is involved in clinical trials sponsored by Endo, FKD, JBL, Genentech, SWOG, URoGen, Vaxiion and Viventia; he serves as a consultant or on the advisory board for Ferring, Genentech, Merck, Pfizer, QED, UroGen, Vaxiion, Verity. E.P. receives institutional funding from Astellas, AstraZeneca, BMS, Genentech, Merck, Peloton, Pfizer; she serves as a consultant for BMS, Genentech, Incyte, Janssen, Merck, AstraZeneca, Pfizer. E.Y.Y. receives institutional funding from Bayer, Blue Earth, Daiichi-Sankyo, Dendreon, Merck, Pharmacyclics, Seattle Genetics, Taiho; he serves as a consultant or on the advisory board for Bayer, Clovis, Merck. M.D.G. holds stock/ownership in Rappta Therapeutics, receives institutional funding from Janssen Oncology, Dendreon, Novartis, BMS, Merck, AstraZeneca, Genentech/Roche; he serves as a consultant for BioMotiv, Janssen, Dendreon, Merck, GlaxoSmithKline, Lilly, Astellas Pharma, Genentech, EMD Serono, AstraZeneca, Seattle Genetics, Incyte, Aileron Therapeutics, Dracen, Inovio Pharmaceuticals, NuMab, Dragonfly Therapeutics. His institution holds a patent in Methods and Compositions for Treating Cancer and Related Methods.
Additional information
Peer review information
Nature Reviews Urology thanks E. Kikuchi, S. Sridhar and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
FDA–BCAN workshop: https://www.fda.gov/drugs/news-events-human-drugs/fda-bcan-workshop-endpoints-development-neoadjuvant-systemic-therapy-regimens-muscle-invasive
Rights and permissions
About this article
Cite this article
Chang, E., Apolo, A.B., Bangs, R. et al. Refining neoadjuvant therapy clinical trial design for muscle-invasive bladder cancer before cystectomy: a joint US Food and Drug Administration and Bladder Cancer Advocacy Network workshop. Nat Rev Urol 19, 37–46 (2022). https://doi.org/10.1038/s41585-021-00505-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-021-00505-w