Abstract
Background
The aim of this study is to investigate of the relationship between GSTM1 gene variations and serum trace elements, plasma malondialdehyde levels in patient with colorectal cancer.
Mateials and Methods.
Genotype distributions of GSTM1 gene variations were determined using real-time polymerase chain reaction method. Serum trace element levels were determined using atomic absorption spectrophotometer method and plasma MDA levels were measurement by spectrophotometric method.
Results
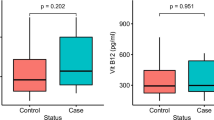
Serum Cu levels, plasma MDA levels and Cu/Zn ratio were determined significantly higher in the group of CRC patient carrying the GA heterozygous genotype of the GSTM1 (rs 112,778,559) gene variation compared to healthy controls (p < 0.05). Serum Cu, Zn levels, plasma MDA levels and Cu/Zn ratio were determined significantly higher in patients carrying GG homozygous genotype of the GSTM1 (rs 112778559) gene variation compared to healthy controls carrying same genotype (p < 0.05). Serum Cu, Zn levels, plasma MDA levels and Cu/Zn ratio were determined significantly higher in the group of CRC patient carrying the GG homozygous genotype of the GSTM1 (rs 12068997) gene variation compared to healthy controls (p < 0.05). On the other hand, serum Se levels were detected significantly lower in CRC patients carrying GA heterozygous and GG homozygous genotypes for GSTM1 (rs 112,778,559) and (rs 12,068,997) gene variations compared to healthy controls (p < 0.05).
Conclusion
In our study, the evaluation of serum Cu, Zn and Se trace element levels and plasma MDA levels according to GSTM1 gene variations genotype distributions were enabled to obtain important biomarkers in terms of CRC development and progression.



Similar content being viewed by others
References
Rašić I, Rašić A, Akšamija G et al (2018) The relationship between serum level of malondialdehyde and progression of colorectal cancer. Acta Clin Croat 57:411–416
Tavassoli E, Reisi M, Javadzad SH et al (2014) The effect of education on the improvement of fruits and vegetables consumption aiming to preventing colorectal cancer. Gastroenterol Hepatol Bed Bench 7(2):94–100
Buyukdogan M, Boruban MC, Artac M et al (2009) Frequency of cytochrome 450 (CYP2C9 and CYP2C19) genetic polymorphisms in patients with colorectal carsinoma. Int J Hematol Oncol 19(3):134–139
Janion K, Szczepańska E, Nowakowska-Zajdel E et al (2020) Selected oxidative stress markers in colorectal cancer patients in relation to primary tumor location-a preliminary research. Medicina (Kaunas). https://doi.org/10.3390/medicina56020047
Song L, Yang C, He X-F (2020) Individual and combined effects of GSTM1 and GSTT1 polymorphisms on colorectal cancer risk: an updated meta-analysis. Biosci Rep 28:40(8):BSR20201927.https://doi.org/10.1042/BSR20201927.
Pacholak LM, Amarante MK, Guembarovski RL et al (2020) Polymorphisms in GSTT1 and GSTM1 genes as possible risk factors for susceptibility to breast cancer development and their influence in chemotherapy response: a systematic review. Mol Biol Rep 47(7):5495–5501
Medjani S, Chellat-Rezgoune D, Kezai T et al (2020) Association of CYP1A1, GSTM1 and GSTT1 gene polymorphisms with risk of prostate cancer in Algerian population. Afr J Urol 26(1):44. https://doi.org/10.1186/s12301-020-00049-2
Albarakati N, Khayyat D, Dallol A et al (2019) The prognostic impact of GSTM1/GSTP1genetic variants in bladder Cancer. BMC Cancer 19(1):991. https://doi.org/10.1186/s12885-019-6244-6
Klusek J, Nasierowska-Guttmejer A, Kowalik A, Wawrzycka I, Lewitowicz P, Chrapek M, Głuszek S (2018) GSTM1, GSTT1, and GSTP1 polymorphisms and colorectal cancer risk in Polish nonsmokers. Oncotarget 9(30):21224–21230. https://doi.org/10.18632/oncotarget.25031
Huang M, Zeng Y, Zhao F, Huang Y (2018) Association of glutathione S-transferase M1 polymorphisms in the colorectal cancer risk: A meta-analysis. J Can Res Ther 14(1):176–183
Wan Rashidi WN, Abu Bakar S (2019) Glutathione S-Transferase: an overview on distribution of GSTM1 and GSTT1 polymorphisms in Malaysian and other populations. Mal J Med Health Sci 15(SP2):85–95
Yetişgin F, Bilici M, Esen R (2021) Evaluation of serum levels of trace elements in myeloproliferative neoplasms: a case-control study. Eastern J Med 26(2):344–350
Popescu E, Stanescu AMA (2019) Trace elements and cancer. Medicina Moderna-Modern Medicine 26(4):169–175
Saleh SAK, Adly HM, Altaf A et al (2020) Serum levels of selenium, zinc, copper, manganese, and iron in prostate cancer patients. Curr Urol 14:44–49
Wang H, Liu H, Zhou M et al (2020) Correlations between 13 trace elements and circulating tumor cells in patients with colorectal cancer in Guangzhou. China Biol Trace Elem Res 198(1):58–67
Osredkar J, Sustar N (2011) Copper and zinc, biological role and significance of copper/zinc imbalance. J Clinic Toxicol S 3:001. https://doi.org/10.4172/2161-0495.S3-001
Ferlay J, Shin HR, Bray F et al (2010) Estimates of worldwide burden of cancer in 2008: Globocan 2008 International journal of cancer. Journal international du cancer 127(12):2893–2917
Cunningham D, Atkin W, Lenz HJ et al (2010) Colorectal cancer. Lancet 375(9719):1030–1047
Markowitz SD, Bertagnolli MM (2009) Molecular origins of cancer: Molecular basis of colorectal cancer. New Engl J Med 361(25):2449–2460
Fearon ER (2011) Molecular genetics of colorectal cancer. Annu Rev Pathol 6:479–507. https://doi.org/10.1146/annurev-pathol-011110-130235
Sánchez-Siles M, Pelegrín-Hernández JP, Hellin-Meseguer D et al (2020) Genotype of null polymorphisms in genes GSTM1, GSTT1, CYP1A1, and CYP1A1*2A (rs4646903T>C) / CYP1A1*2C (rs1048943 A>G) in patients with larynx cancer in southeast Spain. Cancers 12(9):2478. https://doi.org/10.3390/cancers12092478
Miao LF, Ye XH, He XF (2020) Individual and combined effects of GSTM1, GSTT1, and GSTP1 polymorphisms on breast cancer risk: a meta-analysis and re-analysis of systematic meta-analyses. PLoS ONE 15(3):e0216147. https://doi.org/10.1371/journal.pone.0216147
Chirila DN, Chirila MD, Istoan SA et al (2018) The glutathione s-transferases (GSTS) gene polymorphisms in colorectal cancers. Human Veterinary Med 10(2):93–97
Hamachi T, Tajima O, Uezono K et al (2013) CYP1A1, GSTM1, GSTT1 and NQO1 polymorphisms and colorectal adenomas in Japanese men. World J Gastroenterol 19(25):4023–4030. https://doi.org/10.3748/wjg.v19.i25.4023
Klusek J, Nasierowska-Guttmejer A, Kowalik A et al (2019) The influence of red meat on colorectal cancer occurrence is dependent on the genetic polymorphisms of S-glutathione transferase genes. Nutrients 11(7):1682. https://doi.org/10.3390/nu11071682
Kaba M, Pirinççi N, Yüksel MB et al (2015) Serum levels of trace elements in patients with testicular cancers. Int Braz J Urol 41(6):1101–1107. https://doi.org/10.1590/S1677-5538.IBJU.2014.0460
Lossow K, Schwarz M, Kipp AP (2021) Are trace element concentrations suitable biomarkers for the diagnosis of cancer? Redox Biol 42:101900. https://doi.org/10.1016/j.redox.2021.101900
Choi R, Kim MJ, Sohn I et al (2018) Serum trace elements and their associations with breast cancer subgroups in Korean breast cancer patients. Nutrients 11(1):37. https://doi.org/10.3390/nu11010037
Gowri Naidu B, Sarita P, Naga Raju GJ, Tiwari MK (2019) Multivariate analysis of trace elemental data obtained from blood serum of breast cancer patients using SRXRF. Results Phys 12:673–680
Ozmen H, Erulas FA, Karatas F, Cukurovali A, Yalcin O (2006) Comparison of the concentration of trace metals (Ni, Zn Co, Cu and Se), Fe, vitamins A, C and E, and lipid peroxidation in patients with prostate cancer. Clin Chem Lab Med 44(2):175–179. https://doi.org/10.1515/CCLM.2006.032
Zabłocka-Słowińska K, Płaczkowska S, Prescha A, Pawełczyk K, Porębska I, Kosacka M, Pawlik-Sobecka L, Grajeta H (2018) Serum and whole blood Zn, Cu and Mn profiles and their relation to redox status in lung cancer patients. J Trace Elem Med Biol 45:78–84. https://doi.org/10.1016/j.jtemb.2017.09.024
Gecit İ, Kavak S, Meral I, Pirinci N, Güneş M, Demir H et al (2011) Effects of shock waves on oxidative stress, antioxidant enzyme and element levels in kidney of rats. Biol Trace Elem Res 144:1069–1076
Pirincci N, Gecit I, Gunes M, Kaba M, Tanik S, Yuksel MB et al (2013) Levels of serum trace elements in renal cell carcinoma cases. Asian Pac J Cancer Prev 14:499–502
Wu T, Sempos CT, Freudenheim JL, Muti P, Smit E (2004) Serum iron, copper and zinc concentrations and risk of cancer mortality in US adults. Ann Epidemiol 14:195–201
Cunzhi H, Jiexian J, Xianwen Z, Jingang G, Shumin Z, Lili D (2003) Serum and tissue levels of six trace elements and copper/zinc ratio in patients with cervical cancer and uterine myoma. Biol Trace Elem Res 94:113–122
Yaman M, Kaya G, Simsek M (2007) Comparison of trace element concentrations in cancerous and noncancerous human endometrial and ovary tissues. Int J Gynecol Cancer 17:220–228
Karlinskiĭ VM, Bogomolova GG (1985) Change in zinc metabolism in malignant neoplasms. Vopr Onkol 31:25–29
Wang J, Zhao H, Xu Z, Cheng X (2020) Zinc dysregulation in cancers and its potential as a therapeutic target. Cancer Biol Med 17(3):612–625. https://doi.org/10.20892/j.issn.2095-3941.2020.0106
Yang YW, Dai CM, Chen XH, Feng JF (2021) The Relationship between Serum Trace Elements and Oxidative Stress of Patients with Different Types of Cancer. Oxid Med Cell Longev 2021:4846951. https://doi.org/10.1155/2021/4846951
Valko M, Rhodes CJ, Moncol J et al (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160(1):1–40. https://doi.org/10.1016/j.cbi.2005.12.009
Reszka E, Wasowicz W, Gromadzinska J (2007) Antioxidant defense markers modulated by glutathione S-transferase genetic polymorphism: results of lung cancer case-control study. Genes Nutr 2(3):287–294. https://doi.org/10.1007/s12263-007-0057-y
Acknowledgements
Our study was carried out in Trakya University Faculty of Medicine, Department of Biophysics, Department of Medical Oncology and Department of Radiation Oncology.
Funding
This study was supported by the Trakya University Scientific Research Projects Unit (TUBAP) with the project number 2013/14.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [Arzu Ay], [Nevra Alkanli], [Tevfik Gulyasar], [Tammam Sipahi], [Irfan Cicin], [Zafer Kocak] and [Necdet Sut]. The first draft of the manuscript was written by [Arzu Ay and Nevra Alkanli] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. The corresponding author attests that all listed authors meet the authorship criteria and that no other authors meeting the criteria have been omitted.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
For our study, it was applied for Trakya University Faculty of Medicine Non-Invasive Clinical Research Ethics Committee and ethics committee approval was obtained with the protocol code of TÜTF-GOKAEK 2012/198.
Consent for participate
Signed informed consent form was collected from each individual in the patient group with CRC and healthy control group
Consent for publish
Consent for publication is not required as no identifying personal information is published in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ay, A., Gulyasar, T., Alkanli, N. et al. Investigation of the relationship between GSTM1 gene variations and serum trace elements, plasma malondialdehyde levels in patients with colorectal cancer. Mol Biol Rep 48, 6911–6921 (2021). https://doi.org/10.1007/s11033-021-06694-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06694-2