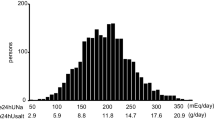
Abstract
Background
Urine sodium and potassium are used as surrogate markers for dietary consumption in adults with hypertension, but their role in youth with hypertension and their association with components of the renin-angiotensin-aldosterone system (RAAS) are incompletely characterized. Some individuals with hypertension may have an abnormal RAAS response to dietary sodium and potassium intake, though this is incompletely described. Our objective was to investigate if plasma renin activity and serum aldosterone are associated with urine sodium and potassium in youth referred for hypertensive disorders.
Methods
This pilot study was a cross-sectional analysis of baseline data from 44 youth evaluated for hypertensive disorders in a Hypertension Clinic. We recorded urine sodium and potassium concentrations normalized to urine creatinine, plasma renin activity, and serum aldosterone and calculated the sodium/potassium (UNaK) and aldosterone/renin ratios. We used multivariable generalized linear models to estimate the associations of renin and aldosterone with urine sodium and potassium.
Results
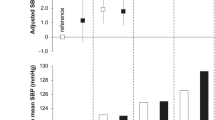
Our cohort was diverse (37% non-Hispanic Black, 14% Hispanic), 66% were male, and median age was 15.3 years; 77% had obesity and 9% had a secondary etiology. Aldosterone was associated inversely with urine sodium/creatinine (β: −0.34, 95% CI −0.62 to −0.06) and UNaK (β: −0.09, 95% CI −0.16 to −0.03), and adjusted for estimated glomerular filtration rate and serum potassium.
Conclusions
Higher serum aldosterone levels, but not plasma renin activity, were associated with lower urine sodium/creatinine and UNaK at baseline in youth referred for hypertensive disorders. Further characterization of the RAAS could help define hypertension phenotypes and guide management.
Graphical abstract

A higher resolution version of the Graphical abstract is available as supplementary information.


Similar content being viewed by others
Availability of data and material
The datasets generated from this study are available from the corresponding author upon reasonable request.
Code availability
The code used to analyze the data from this study is available from the corresponding author upon reasonable request.
References
Flynn J (2013) The changing face of pediatric hypertension in the era of the childhood obesity epidemic. Pediatr Nephrol 28:1059–1066. https://doi.org/10.1007/s00467-012-2344-0
Feber J, Ahmed M (2010) Hypertension in children: new trends and challenges. Clin Sci 119:151–161. https://doi.org/10.1042/CS20090544
Frisancho AR, Leonard WR, Bollettino LA (1984) Blood pressure in blacks and whites and its relationship to dietary sodium and potassium intake. J Chronic Dis 37:515–519. https://doi.org/10.1016/0021-9681(84)90002-X
Ganguli M (1997) Higher education and income are related to a better Na:K ratio in Blacks baseline results of the Treatment of Mild Hypertension Study (TOMHS) data. Am J Hypertens 10:979–984. https://doi.org/10.1016/S0895-7061(97)00162-3
Walker WG, Whelton PK, Saito H, Russell RP et al (1979) Relation between blood pressure and renin, renin substrate, angiotensin II, aldosterone and urinary sodium and potassium in 574 ambulatory subjects. Hypertension 1:287–291. https://doi.org/10.1161/01.HYP.1.3.287
Lee S-G, Lee W, Kwon OH, Kim J-H (2013) Association of urinary sodium/creatinine ratio and urinary sodium/specific gravity unit ratio with blood pressure and hypertension: KNHANES 2009–2010. Clin Chim Acta 424:168–173. https://doi.org/10.1016/j.cca.2013.05.027
South AM, Arguelles L, Finer G, Langman CB (2017) Race, obesity, and the renin-angiotensin-aldosterone system: treatment response in children with primary hypertension. Pediatr Nephrol 32:1585–1594. https://doi.org/10.1007/s00467-017-3665-9
Ibrahim MM (2006) RAS inhibition in hypertension. J Hum Hypertens 20:101–108. https://doi.org/10.1038/sj.jhh.1001960
Ljungman S, Aurell M, Hartford M, Wilhelmsen L et al (1981) Sodium excretion and blood pressure. Hypertension 3:318–326. https://doi.org/10.1161/01.HYP.3.3.318
Pietinen PI, Wong O, Altschul AM (1979) Electrolyte output, blood pressure, and family history of hypertension. Am J Clin Nutr 32:997–1005
Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D et al (2017) Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 140:e20171904. https://doi.org/10.1542/peds.2017-1904 Erratum in 140:e20173035
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2004) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114:555–576
Harris PA, Taylor R, Minor BL, Elliot V et al (2019) The REDCap consortium: building an international community of software platform partners. J Biomed Inform 95:103208. https://doi.org/10.1016/j.jbi.2019.103208
Barlow SE (2007) Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 120:S164–S192. https://doi.org/10.1542/peds.2007-2329C
Rosner B, Cook N, Portman R, Daniels S et al (2008) Determination of blood pressure percentiles in normal-weight children: some methodological issues. Am J Epidemiol 167:653–666. https://doi.org/10.1093/aje/kwm348
South AM, Palakshappa D, Brown CL (2019) Relationship between food insecurity and high blood pressure in a national sample of children and adolescents. Pediatr Nephrol 34:1583–1590. https://doi.org/10.1007/s00467-019-04253-3
Chappell MC (2016) Biochemical evaluation of the renin-angiotensin system: the good, bad, and absolute? Am J Physiol Heart Circ Physiol 310:H137–H152. https://doi.org/10.1152/ajpheart.00618.2015
South AM, Nixon PA, Chappell MC, Diz DI et al (2018) Association between preterm birth and the renin−angiotensin system in adolescence: influence of sex and obesity. J Hypertens 36:2092–2101. https://doi.org/10.1097/HJH.0000000000001801
Sparks MA, South AM, Badley AD, Baker-Smith CM et al (2020) Severe acute respiratory syndrome coronavirus 2, COVID-19, and the renin-angiotensin system: pressing needs and best research practices. Hypertension 76:1350–1367. https://doi.org/10.1161/HYPERTENSIONAHA.120.15948
Schwartz GJ, Brion LP, Spitzer A (1987) The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin N Am 34:571–590. https://doi.org/10.1016/S0031-3955(16)36251-4
Croghan C, Egeghy PP (2003) Methods of dealing with values below the limit of detection using SAS. Presented at Southeastern SAS User Group, St. Petersburg, FL, September 22-24, 2003. https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NERL&dirEntryId=64046
Cohen JB, D’Agostino McGowan L, Jensen ET, Rigdon J et al (2021) Evaluating sources of bias in observational studies of angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker use during coronavirus disease 2019: beyond confounding. J Hypertens 39:795–805. https://doi.org/10.1097/HJH.0000000000002706
Jain N, Minhajuddin AT, Neeland IJ, Elsayed EF et al (2014) Association of urinary sodium-to-potassium ratio with obesity in a multiethnic cohort. J Clin Nutr 99:992–998. https://doi.org/10.3945/ajcn.113.077362
Langford HG (1983) Dietary potassium and hypertension: epidemiologic data. Ann Intern Med 98:770. https://doi.org/10.7326/0003-4819-98-5-770
Shrier I, Platt RW (2008) Reducing bias through directed acyclic graphs. BMC Med Res Methodol 8:70. https://doi.org/10.1186/1471-2288-8-70
Martinez-Aguayo AG, Campino C, Rodriguez-Fernandez M, Poggi H et al (2020) Urinary sodium-to-potassium ratio and plasma renin and aldosterone concentrations in normotensive children: implications for the interpretation of results. J Hypertens 38:671–678. https://doi.org/10.1097/HJH.0000000000002324
Drenjančević-Perić I, Jelaković B, Lombard JH, Kunert MP et al (2011) High-salt diet and hypertension: focus on the renin-angiotensin system. Kidney Blood Press Res 34:1–11. https://doi.org/10.1159/000320387
Mizelle HL, Montani JP, Hester RL, Didlake RH et al (1993) Role of pressure natriuresis in long-term control of renal electrolyte excretion. Hypertension 22:102–110. https://doi.org/10.1161/01.hyp.22.1.102
Gonzalez-Campoy JM, Romero JC, Knox FG (1989) Escape from the sodium-retaining effects of mineralocorticoids: role of ANF and intrarenal hormone systems. Kidney Int 35:767–777. https://doi.org/10.1038/ki.1989.51
Majid D, Prieto M, Navar L (2015) Salt-sensitive hypertension: perspectives on intrarenal mechanisms. Curr Hypertens Rev 11:38–48. https://doi.org/10.2174/1573402111666150530203858
Lava SAG, Bianchetti MG, Simonetti GD (2015) Salt intake in children and its consequences on blood pressure. Pediatr Nephrol 30:1389–1396. https://doi.org/10.1007/s00467-014-2931-3
(2012) Guideline: sodium intake for adults and children. Geneva, World Health Organization. https://www.ncbi.nlm.nih.gov/books/NBK133309/
Gowrishankar M, Blair B, Rieder MJ (2020) Dietary intake of sodium by children: why it matters. Paediatr Child Health 25:47–53. https://doi.org/10.1093/pch/pxz153
Rayner BL, Owen EP, King JA, Soule JA et al (2003) A new mutation, R563Q, of the beta subunit of the epithelial sodium channel associated with low-renin, low-aldosterone hypertension. J Hypertens 21:921–926. https://doi.org/10.1097/00004872-200305000-00016
Saccomani G, Mitchell KD, Navar LG (1990) Angiotensin II stimulation of Na(+)-H+ exchange in proximal tubule cells. Am J Phys 258:F1188–F1195. https://doi.org/10.1152/ajprenal.1990.258.5.F1188
Dibo P, Marañón RO, Chandrashekar K, Mazzuferi GB et al (2019) Angiotensin-(1-7) inhibits sodium transport via Mas receptor by increasing nitric oxide production in thick ascending limb. Phys Rep 7:e14015. https://doi.org/10.14814/phy2.14015
South AM, Brady TM, Flynn JT (2020) ACE2 (angiotensin-converting enzyme 2), COVID-19, and ACE inhibitor and Ang II (angiotensin II) receptor blocker use during the pandemic: the pediatric perspective. Hypertension 76:16–22. https://doi.org/10.1161/HYPERTENSIONAHA.120.15291
South AM, Shaltout HA, Washburn LK, Hendricks AS et al (2019) Fetal programming and the angiotensin-(1-7) axis: a review of the experimental and clinical data. Clin Sci 133:55–74. https://doi.org/10.1042/CS20171550
South AM, Diz DI, Chappell MC (2020) COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol 318:H1084–H1090. https://doi.org/10.1152/ajpheart.00217.2020
Faulkner JL, Harwood D, Bender L, Shrestha L et al (2018) Lack of suppression of aldosterone production leads to salt-sensitive hypertension in female but not male Balb/C mice. Hypertension 72:1397–1406. https://doi.org/10.1161/HYPERTENSIONAHA.118.11303
Di Giosia P, Giorgini P, Stamerra CA, Petrarca M et al (2018) Gender differences in epidemiology, pathophysiology, and treatment of hypertension. Curr Atheroscler Rep 20:13. https://doi.org/10.1007/s11883-018-0716-z
Reckelhoff JF (2001) Gender differences in the regulation of blood pressure. Hypertension 37:1199–1208. https://doi.org/10.1161/01.HYP.37.5.1199
Xue B, Johnson AK, Hay M (2013) Sex differences in angiotensin II- and aldosterone-induced hypertension: the central protective effects of estrogen. Am J Phys Regul Integr Comp Phys 305:R459–R463. https://doi.org/10.1152/ajpregu.00222.2013
South AM, Nixon PA, Chappell MC, Diz DI et al (2019) Renal function and blood pressure are altered in adolescents born preterm. Pediatr Nephrol 34:137–144. https://doi.org/10.1007/s00467-018-4050-z
Monteonofrio L, Florio MC, AlGhatrif M, Lakatta EG et al (2021) Aging- and gender-related modulation of RAAS: potential implications in COVID-19 disease. Vasc Biol 3:R1–R14. https://doi.org/10.1530/VB-20-0014
Pratt JH, Manatunga AK, Bloem LJ, Li W (1993) Racial differences in aldosterone excretion: a longitudinal study in children. J Clin Endocrinol Metab 77:1512–1515. https://doi.org/10.1210/jcem.77.6.8263135
Tu W, Eckert GJ, Hannon TS, Liu H et al (2014) Racial differences in sensitivity of blood pressure to aldosterone. Hypertension 63:1212–1218. https://doi.org/10.1161/HYPERTENSIONAHA.113.02989
(2021) Pediatric Hypertension and the Renin-Angiotensin SystEm (PHRASE). https://clinicaltrials.gov/ct2/show/NCT04752293. Accessed 12 Feb 2021
(2019) Uric acid, klotho and salt sensitivity in young adults born preterm (PEPC3). https://clinicaltrials.gov/ct2/show/NCT04026776. Accessed 02 Nov 2020
Acknowledgements
We would like to thank Victoria Giammattei, BA, Weston Colbaugh, BS, Lauren Valloy, and the rest of the hypertension registry study team for their assistance with data collection, and Caroline Lucas, BA, for managing the study.
Funding
This study was funded by the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases T35 DK007400 to EP; National Heart, Lung, and Blood Institute K23 HL148394, L40 HL148910, and R01 HL146818 to AMS; National Center for Advancing Translational Sciences UL1 TR001420 to the Wake Forest Clinical and Translational Science Award); the Wake Forest Maya Angelou Center for Health Equity; and the Wake Forest School of Medicine Department of Pediatrics.
Author information
Authors and Affiliations
Contributions
Ms. Perrin conceptualized the research question, collected the data, visualized the data and assisted with data interpretation, drafted the initial manuscript, and reviewed and revised the manuscript. Dr. South conceptualized and designed the study, supervised data collection, analyzed and interpreted the data, created the figures, at all times had full access to and takes full responsibility for the data, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable fully for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval
The Wake Forest School of Medicine Institutional Review Board approved this study (IRB00046001).
Consent to participate
Written informed consent was obtained from parents/guardians and written assent from participants aged 7 years and older in the prospective cohort; the retrospective cohort was deemed exempt from requiring informed consent/assent by the IRB.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PPTX 310 kb)
Rights and permissions
About this article
Cite this article
Perrin, E.C., South, A.M. Correlation between kidney sodium and potassium handling and the renin-angiotensin-aldosterone system in children with hypertensive disorders. Pediatr Nephrol 37, 633–641 (2022). https://doi.org/10.1007/s00467-021-05204-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-021-05204-7