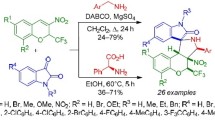
A regio- and stereoselective method for the synthesis of (trifluoro(trichloro)methyl)-substituted spiro[chromeno(thia)pyrrolizidineoxindoles] in 53–97% yields was developed on the basis of the three-component reaction of 3-nitro-2-(trifluoro(trichloro)methyl)-2Hchromenes with azomethine ylides generated in situ from isatins and L-(thia)proline in EtOH at 30°C. The obtained compounds demonstrated high cytotoxic activity against HeLa human cervical cancer and RD human embryonic rhabdomyosarcoma cells.



Similar content being viewed by others
References
(a) Korotaev, V. Yu.; Kutyashev, I. B.; Barkov, A. Yu.; Sosnovskikh, V. Ya. Russ. Chem. Rev. 2019, 88, 27. [Usp. Khim. 2019, 88, 27.] (b) Vroemans, R.; Dehaen, W. In Targets in Heterocyclic Systems; Attanasi, O. A.; Merino, P.; Spinelli, D., Eds.; Società Chimica Italiana: Rome, 2018, Vol. 22, p. 318. b Korotaev, V. Yu.; Sosnovskikh, V. Ya.; Barkov, A. Yu. Russ. Chem. Rev. 2013, 82, 1081. [Usp. Khim. 2013, 82, 1081.]
(a) Pratap, R.; Ram, V. J. Chem. Rev. 2014, 114, 10476. (b) O'Connor, C. J.; Beckmann, H. S. G.; Spring, D. R. Chem. Soc. Rev. 2012, 41, 4444. (c) Horton, D. A.; Bourne, G. T.; Smythe, M. L. Chem. Rev. 2003, 103, 893. (d) Costa, M.; Dias, T. A.; Brito, A.; Proença, F. Eur. J. Med. Chem. 2016, 123, 487.
(a) Elomri, A.; Skaltsounis, A.-L.; Michel, S.; Tillequin, F.; Koch, M.; Rolland, Y.; Pierré, A.; Atassi, G. Chem. Pharm. Bull. 1996, 44, 2165. (b) Han, K.; Xu, X.; Chen, G.; Zeng, Y.; Zhu, J.; Du, X.; Zhang, Z.; Cao, B.; Liu, Z.; Mao, X. J. Hematol. Oncol. 2014, 7, 9. (c) Jean, M.; Fouque, A.; Legembre, P.; van de Weghe, P. EP Patent 2853530A1. (d) Wang, S.; Li, J.; Du, Y.; Xu, Y.; Wang, Y.; Zhang, Z.; Xu, Z.; Zeng, Y.; Mao, X.; Cao, B. J. Farmacol. Sci. 2017, 134, 197. (e) Fouqué, A.; Delalande, O.; Jean, M.; Castellano, R.; Josselin, E.; Malleter, M.; Shoji, K. F.; Hung, M. D.; Rampanarivo, H.; Collette, Y.; van de Weghe, P.; Legembre, P. J. Med. Chem. 2015, 58, 6559.
(a) Hartmann, T.; Witte, L. In Alkaloids: Chemical and Biological Perspectives; Pelletier, S. W., Ed.; Pergamon: Oxford, 1995, Vol. 9, p. 155. (b) Bull, L. В.; Culvenor, C. C. J.; Dick, A. Т. The Pyrrolizidine Alkaloids: Their Chemistry, Pathogenicity and Other Biological Properties; John Wiley & Sons: New York, 1968. (c) Robertson, J.; Stevens, K. Nat. Prod. Rep. 2017, 34, 62. (d) Cakmak, M.; Mayer, P.; Trauner, D. Nat. Chem. 2011, 3, 543. (e) Becker, D. P.; Flynn, D. L.; Moormann, A. E.; Nosal, R.; Villamil, C. I.; Loeffler, R.; Gullikson, G. W.; Moummi, C.; Yang, D.-C. J. Med. Chem. 2006, 49, 1125. (f) Miyano, S.; Sumoto, K.; Satoh, F.; Shima, K.; Hayashimatsu, M.; Morita, M.; Aisaka, K.; Noguchi, T. J. Med. Chem. 1985, 28, 714. (g) Sakai, R.; Inoue, D.; Ishibashi, K.; Inoue, M.; Shirayama, T.; Asayama, J.; Nakagawa, M. J. Cardiovasc. Farmacol. 1995, 25, 953.
(a) Boddy, A. J.; Bull, J. A. Org. Chem. Front. 2021, 8, 1026. (b) Izmest'ev, A. N.; Gazieva, G. А.; Kravchenko, A. N. Chem. Heterocycl. Compd. 2020, 56, 255. [Khim. Geterotsikl. Soedin. 2020, 56, 255.] (c) Gataullin, R. R. Helv. Chim. Acta 2020, 103, e2000137. c Nájera, C.; Sansano, J. M. Pure Appl. Chem. 2019, 91, 575.
(a) Yu, B.; Yu, D.-Q.; Liu, H.-M. Eur. J. Med. Chem. 2015, 97, 673. (b) Ding, K.; Lu, Y.; Nikolovska-Coleska, Z.; Qiu, S.; Ding, Y.; Gao, W.; Stuckey, J.; Roller, P. P.; Tomita, Y.; Deschamps, J. R.; Wang, S. J. Am. Chem. Soc. 2005, 127, 10130. (c) Ding, K.; Lu, Y.; Nikolovska-Coleska, Z.; Wang, G.; Qiu, S.; Shangary, S.; Gao, W.; Qin, D.; Stuckey, J.; Krajewski, K.; Roller, P. P.; Wang, S. J. Med. Chem. 2006, 49, 3432. (d) Ivanenkov, Y. A.; Vasilevski, S. V.; Beloglazkina, E. K.; Kukushkin, M. E.; Machulkin, A. E.; Veselov, M. S.; Chufarova, N. V.; Chernyagina, E. S.; Vanzcool, A. S.; Zyk, N. V.; Skvortsov, D. A.; Khutornenko, A. A.; Rusanov, A. L.; Tonevitsky, A. G.; Dontsova, O. A.; Majouga, A. G. Bioorg. Med. Chem. Lett. 2015, 25, 404. (e) Wang, S.; Sun, W.; Zhao, Y.; McEachern, D.; Meaux, I.; Barrière, C.; Stuckey, J. A.; Meagher, J. L.; Bai, L.; Liu, L.; Hoffman-Luca, C. G.; Lu, J.; Shangary, S.; Yu, S.; Bernard, D.; Aguilar, A.; Dos-Santos, O.; Besret, L.; Guerif, S.; Pannier, P.; Gorge-Bernat, D.; Debussche, L. Cancer Res. 2014, 74, 5855.
(a) Arumugam, N.; Kumar, R. S.; Almansour, A. I.; Perumal, S. Curr. Org. Chem. 2013, 17, 1929. (b) Döndas, H. A.; Retamosa, M. G.; Sansano, J. M. Synthesis 2017, 2819. (c) Korotaev, V. Yu.; Zimnitskiy, N. S.; Barkov, A. Yu.; Kutyashev, I. B.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2018, 54, 905. [Khim. Geterotsikl. Soedin. 2018, 54, 905.] (d) Singh, R.; Bhardwaj, D.; Saini, M. R. RSC Adv. 2021, 11, 4760. d Arumugam, N.; Almansour, A. I.; Kumar, R. S.; Ali Al-Aizari, A. J. M.; Alaqeel, S. I.; Kansız, S.; Krishna,V. S.; Sriram, D.; Dege, N. RSC Adv. 2020, 10, 23522. e Rao, M. P.; Gunaga, S. S.; Zuegg, J.; Pamarthi, R.; Ganesh, M. Org. Biomol. Chem. 2019, 17, 9390. f Chornous, V. A.; Mel'nik, O. Ya.; Mel'nik, D. A.; Rusanov, E. B.; Vovk, M. V. Russ. J. Org. Chem. 2015, 51, 1423. [Zh. Org. Khim. 2015, 51, 1454.] (h) Bakthadoss, M.; Sivakumar, N.; Devaraj, A.; Sharada, D. S. Synthesis 2011, 2136.
Kutyashev, I. B.; Ulitko, M. V.; Barkov, A. Yu.; Zimnitskiy, N. S.; Korotaev, V. Yu.; Sosnovskikh, V. Ya. New J. Chem. 2019, 43, 18495.
Korotaev, V. Yu.; Barkovskiy, S V.; Kutyashev, I. B.; Ulitko, M. V.; Barkov, A. Yu.; Zimnitskiy, N. S.; Kochnev, I. A.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2021, 57, 679. [Khim. Geterotsikl. Soedin. 2021, 57, 679.]
(a) Kutyashev, I. B.; Barkov, A. Yu.; Korotaev, V. Yu.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2019, 55, 529. [Khim. Geterotsikl. Soedin. 2019, 55, 529.] (b) Korotaev, V. Yu.; Kutyashev, I. B.; Barkov, A. Yu.; Rozhkova, Yu. S.; Plekhanova, I. V.; Shklyaev, Yu. V.; Sosnovskikh, V. Ya. Tetrahedron Lett. 2019, 60, 150916. b Kutyashev, I. B.; Barkov, A. Yu.; Zimnitskiy, N. S.; Korotaev, V. Yu.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2019, 55, 861. [Khim. Geterotsikl. Soedin. 2019, 55, 861.] (d) Kutyashev, I. B.; Kochnev, I. А.; Cherepkova, A. А.; Zimnitskiy, N. S.; Barkov, A. Yu; Korotaev, V. Yu.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2020, 56, 1302. [Khim. Geterotsikl. Soedin. 2020, 56, 1302.] (e) Kutyashev, I. B.; Sannikov, M. S.; Kochnev, I. A.; Barkov, A. Yu.; Zimnitskiy, N. S.; Korotaev, V. Yu.; Sosnovskikh, V. Ya. SynOpen 2021, 5, 1.
Rao, J. N. S.; Raghunathan, R. Tetrahedron Lett. 2013, 54, 6568.
Nayak, S.; Mishra, S. K.; Bhakta, S.; Panda, P.; Baral, N.; Mohapatra, S.; Purohit, C. S.; Satha, P. Lett. Org. Chem. 2016, 13, 11.
(a) Grigg, R.; Idle, J.; McMeekin, P.; Surendrakumar, S.; Vipond, D. J. Chem. Soc., Perkin Trans. 1 1988, 2703. (b) Toma, Y.; Kunigami, M.; Watanabe, K.; Higashi, M.; Arimitsu, S. J. Fluorine Chem. 2016, 189, 22. (c) Sobhi, C.; Nacereddine, A. K.; Djerourou, A.; Ríos-Gutiérrez, M; Domingo, L. R. J. Phys. Org. Chem. 2017, 30, e3637.
Korotaev, V. Yu.; Barkov, A. Yu.; Moshkin, V. S.; Matochkina, E. G.; Kodess, M. I.; Sosnovskikh, V. Ya. Tetrahedron 2013, 69, 8602.
Wall, M. E.; Wani, M. C.; Cook, C. E.; Palmer, K. H.; McPhail, A. T.; Sim, G. A. J. Am. Chem. Soc. 1966, 88, 3888.
Korotaev, V. Yu.; Kutyashev, I. B.; Sosnovskikh, V. Ya. Heteroat. Chem. 2005, 16, 492.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Mosmann, T. J. Immunol. Methods 1983, 65, 55.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2021, 57(7/8), 751–763
Supplementary Information
ESM 1
(PDF 5905 kb)
Rights and permissions
About this article
Cite this article
Kutyashev, I.B., Ulitko, M.V., Barkov, A.Y. et al. Regio- and Stereoselective 1,3-dipolar Cycloaddition of Azomethine Ylides Based on Isatins and (thia)proline to 3-nitro-2-(trifluoro(trichloro)methyl)-2H-chromenes: Synthesis and Cytotoxic Activity of 6-(trihalomethyl)-spiro[chromeno(thia)pyrrolizidine-11,3'-indolin]-2'-ones. Chem Heterocycl Comp 57, 751–763 (2021). https://doi.org/10.1007/s10593-021-02979-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-021-02979-3