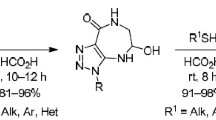
4-(N-Boc-amino)-1Н-1,2,3-triazolecarbothioamides, obtained by a sequential treatment of 4-(N-Boc-amino)-1Н-1,2,3-triazoles with n-BuLi and alkyl isothiocyanates at –78÷–60°C, reacted with ethyl bromoacetate, forming the respective 4-(N-Boc-amino)-5-thioimidates, which underwent intramolecular cyclocondensation upon treatment in saturated НCl solution in dioxane, producing 8-(alkylimino)-4,8-dihydro-1Н-[1,2,3]triazolo[4,5-e][1,4]thiazepin-5(6Н)-ones.

Similar content being viewed by others
References
Chiesi, M.; Schwaller, R.; Eickenberger, K. Biochem. Pharmacol. 1988, 37, 4399.
Malli, R.; Frieden, M.; Trenker, M.; Graier, W. F. J. Biol. Chem. 2005, 280, 12114.
Pei, Y.; Lilly, M. J.; Owen, D. J.; D’Souza, L. J.; Tang, X.-Q.; Yu, J.; Nazarbaghi, R.; Hunter, A.; Anderson, C. M.; Glasco, S.; Ede, N. J.; James, I. W.; Maitra, U.; Chandrasekaran, S.; Moos, W. H.; Ghosh, S. S. J. Org. Chem. 2003, 68, 92.
Martínez-Sanz, F. J.; Lajarín-Cuesta, R.; González-Lafuente, L.; Moreno-Ortega, A. I.; Punzón, E.; Cano-Abad, M. F. Eur. J. Med. Chem. 2016, 109, 114.
Moreno-Ortega, A. I.; Martínez-Sanz, F. J.; Lajarín-Cuesta, R.; De Los Ríos, C.; Cano-Abad, M. F. Neuropharmacology 2015, 95, 503.
Garofalo, A.; Campiani, G.; Fiorini, I.; Nacci, V. Farmaco 1993, 48, 275.
Wu, L.; Yang. X.; Peng, Q.; Sun, G. Eur. J. Med. Chem. 2017, 127, 599.
Miki, T.; Kori, M.; Fujishima, A.; Mabuchi, H.; Tozawa, R.; Nakamura, M.; Sugiyama, Y.; Yukimasa, H. Bioorg. Med. Chem. 2002, 10, 385.
Marinozzi, M.; Carotti, A.; Sansone, E.; Macchiarulo, A.; Rosatelli, E.; Sardella, R.; Natalini, B.; Rizzo, G.; Adorini, L.; Passeri, D.; De Franco, F.; Pruzanski, M.; Pellicciari, R. Bioorg. Med. Chem. 2012, 20, 3429.
Marinozzi, M.; Carotti, A.; Sardella, R.; Buonerba, F.; Ianni, F.; Natalini, B.; Passeri, D.; Rizzo, G.; Pellicciari, R. Bioorg. Med. Chem. 2013, 21, 3780.
Shi, F.; Zeng, X.-N.; Cao, X.-D.; Zhang, S.; Jiang, B.; Zheng, W.-F.; Tu, S.-J. Bioorg. Med. Chem. Lett. 2012, 22, 743.
Zhang, L.-L.; Li, Y.-T.; Gao, T.; Guo, S.-S.; Yang, B.; Meng, Z.-H.; Dai, Q.-P.; Xu, Z.-B.; Wu, Q.-P. Synthesis 2019, 4170.
Dheer, D.; Singh, V.; Shankar, R. Bioorg. Chem. 2017, 71, 30.
Lal, K.; Yadav, P. Anti-Сancer Agents Med. Chem. 2018, 18, 21.
Kumar, S.; Sharma, B.; Mehra, V.; Kumar, V. Eur. J. Med. Chem. 2021, 212, 113069.
Bozorov, K.; Zhao, J.; Aisa, H. A. Bioorg. Med.Chem. 2019, 27, 3511.
Gonzaga, D. T. G.; da Rocha, D. R.; da Silva, F. C.; Ferreira, V. F. Curr. Top. Med. Chem. 2013, 13, 2850.
Tu, S.-J.; Cao, X.-D.; Hao, W.-J.; Zhang, X.-H.; Yan, S.; Wu, S.-S.; Han, Z.-G.; Shi, F. Org. Biomol. Chem. 2009, 7, 557.
Drawanz, B. B.; Zimmer, G. C.; Rodrigues, L. V.; Nörnberg, A. B.; Horner M; Frizzo, C. P.; Cunico, W. Synthesis 2017, 5167.
Saini, R.; Malladi, S. R.; Dharavath, N. J. Heterocycl. Chem. 2018, 55, 1579.
Anisetti, R.; Reddy, M. S. J. Sulfur Chem. 2012, 33, 363.
Joshi, K. C.; Pathak, V. N.; Gard, U. J. Heterocycl. Chem. 1980, 17, 789.
Chen, H.; Shi, D. Tetrahedron 2011, 67, 5686.
Karnakar, K.; Murthy, S. N.; Ramesh, K.; Reddy, K. H. V.; Nageswar, Y. V. D.; Chandrakala, U.; Prabhavathi Devi, B. L. A.; Prasad, R. B. N. Tetrahedron Lett. 2012, 53, 3497.
Dandia, A.; Singh, R.; Joshi, J.; Maheshwari, S.; Soni, P. RSC Adv. 2013, 3, 18992.
Zhang, F.; Li, C.; Qi, C. RSC Adv. 2016, 6, 102924.
Zaleska, B.; Cież, D.; Grochowski, J.; Serda, P.; Winnik, W. Liebigs Ann. 1996, 1996, 211.
Greenhill, J. V.; Hanaee, J.; Steel. P. J. J. Chem. Soc., Perkin Trans. 1 1990, 1869.
Augustin, M.; Köhler, H. J. Prakt. Chem. 1984, 326, 401.
Gros, L.; Wesolowska, A.; Westerlich, S.; Jagodzinski, T. J. Heterocycl. Chem. 2007, 44, 167.
Syrota, N. A.; Kemskiy, S. V.; Bol'but, A. V.; Vovk, M. V. Chem. Heterocycl. Compd. 2019, 55, 1092. [Khim. Geterotsikl. Soedin. 2019, 55, 1092.]
Syrota, N. A.; Kemskiy, S. V.; Bol'but, A. V.; Chernobaev, I. I.; Vovk, M. V. Chem. Heterocycl. Compd. 2020, 56, 1048. [Khim. Geterotsikl. Soedin. 2020, 56, 1048.]
Jagodzinski, T. S.; Dziembowska, T.; Jagodzinska, E.; Rozvadovski, Z. Pol. J. Chem. 2001, 75, 1853.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2021, 57(7/8), 841–847
Rights and permissions
About this article
Cite this article
Syrota, N.A., Kemskiy, S.V., Bol’but, A.V. et al. 4-(N-Boc-amino)-1Н-1,2,3-triazolecarbothioamides in the synthesis of a new heterocyclic [1,2,3]triazolo[4,5-e][1,4]thiazepine system. Chem Heterocycl Comp 57, 841–847 (2021). https://doi.org/10.1007/s10593-021-02989-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-021-02989-1