Abstract—
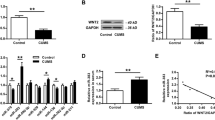
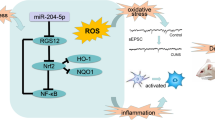
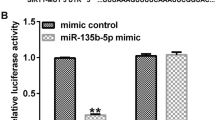
Depression is a major threat to global mental health and demands targeted therapeutic regimens. The current study set out to evaluate the regulatory mechanism of nuclear factor erythroid-2 related factor 2 (Nrf2) in depression-induced cognitive dysfunction and inflammatory injury. First, depressive rat models were established via chronic unpredicted mild stress (CUMS) treatment. Cognitive function of rats was assessed by a series of behavioral tests. Rats were further stereotactically injected with Nrf2 overexpression vector, with expression patterns of Nrf2, miR-17-5p, and wolfram syndrome 1 (Wfs1) detected using qRT-PCR and Western blot assay. In addition, pathological changes of murine hippocampus were analyzed using hematoxylin–eosin staining. In vitro cell models were additionally established using lipopolysaccharide. Cell viability was detected via the CCK-8 method. Moreover, levels of TNF-α, IL-1β, and IL-10 were detected via ELISA. Furthermore, the binding relationships between Nrf2 and the miR-17-5p promoter, miR-17-5p, and Wfs1 were verified. It was found that Nrf2 was weakly expressed in CUMS-treated rats, whereas Nrf2 upregulation alleviated cognitive dysfunction and brain inflammatory injury. Meanwhile, Nrf2 inhibited miR-17-5p expression via binding to the miR-17-5p promoter. miR-17-5p was also found to limit Wfs1 transcription. miR-17-5p overexpression or Wfs1 downregulation partly reversed the role of Nrf2 in reliving inflammatory injury of murine hippocampal neurons. Overall, our findings indicated that Nrf2 inhibited miR-17-5p expression and promoted Wfs1 transcription, thereby alleviating cognitive dysfunction and inflammatory injury in rats with depression-like behaviors.









Similar content being viewed by others
References
Dean, J., and M. Keshavan. 2017. The neurobiology of depression: An integrated view. Asian Journal of Psychiatry 27: 101–111.
Michaelides, A., and P. Zis. 2019. Depression, anxiety and acute pain: Links and management challenges. Postgraduate Medicine 131: 438–444.
Culpepper, L., R.W. Lam, and R.S. McIntyre. 2017. Cognitive impairment in patients with depression: Awareness, assessment, and management. Journal of Clinical Psychiatry 78: 1383–1394.
Pandarakalam, J.P. 2018. Challenges of treatment-resistant depression. Psychiatria Danubina 30: 273–284.
Malhi, G.S., and J.J. Mann. 2018. Depression. Lancet 392: 2299–2312.
Li Y., T. Cao, R.M. Ritzel, J. He, A.I. Faden and J. Wu. 2020. Dementia, depression, and associated brain inflammatory mechanisms after spinal cord injury. Cells 9.
do Espirito Santo C.C., F. da Silva Fiorin, J. Ilha, M. Duarte, T. Duarte and A.R.S. Santos. 2019. Spinal cord injury by clip-compression induces anxiety and depression-like behaviours in female rats: The role of the inflammatory response. Brain, Behavior, and Immunity 78: 91–104.
Hashimoto, K. 2018. Essential Role of Keap1-Nrf2 Signaling in mood disorders: Overview and future perspective. Frontiers in Pharmacology 9: 1182.
Qu, Y., J. Shan, S. Wang, L. Chang, Y. Pu, X. Wang, Y. Tan, M. Yamamoto, and K. Hashimoto. 2021. Rapid-acting and long-lasting antidepressant-like action of (R)-ketamine in Nrf2 knock-out mice: A role of TrkB signaling. European Archives of Psychiatry and Clinical Neuroscience 271: 439–446.
Nakayama, T., K. Okimura, J. Shen, Y.J. Guh, T.K. Tamai, A. Shimada, S. Minou, Y. Okushi, T. Shimmura, Y. Furukawa, N. Kadofusa, A. Sato, T. Nishimura, M. Tanaka, K. Nakayama, N. Shiina, N. Yamamoto, A.S. Loudon, T. Nishiwaki-Ohkawa, A. Shinomiya, T. Nabeshima, Y. Nakane, and T. Yoshimura. 2020. Seasonal changes in NRF2 antioxidant pathway regulates winter depression-like behavior. Proceedings of the National Academy of Sciences of the United States of America 117: 9594–9603.
Yao, W., S. Lin, J. Su, Q. Cao, Y. Chen, J. Chen, Z. Zhang, K. Hashimoto, Q. Qi, and J.C. Zhang. 2021. Activation of BDNF by transcription factor Nrf2 contributes to antidepressant-like actions in rodents. Translational Psychiatry 11: 140.
Kong, Y., L. Hu, K. Lu, Y. Wang, Y. Xie, L. Gao, G. Yang, B. Xie, W. He, G. Chen, H. Wu, X. Wu, F. Zhan, and J. Shi. 2019. Ferroportin downregulation promotes cell proliferation by modulating the Nrf2-miR-17-5p axis in multiple myeloma. Cell Death & Disease 10: 624.
Huang, N., W. Li, X. Wang, and S. Qi. 2018. MicroRNA-17-5p aggravates lipopolysaccharide-induced injury in nasal epithelial cells by targeting Smad7. BMC Cell Biology 19: 1.
Camkurt, M.A., S. Acar, S. Coskun, M. Gunes, S. Gunes, M.F. Yilmaz, A. Gorur, and L. Tamer. 2015. Comparison of plasma MicroRNA levels in drug naive, first episode depressed patients and healthy controls. Journal of Psychiatric Research 69: 67–71.
Yang J., C. Chen, X. Jin, L. Liu, J. Lin, X. Kang and S. Zhu. 2020. Wfs1 and related molecules as key candidate genes in the hippocampus of depression. Frontier Genetics 11: 589370.
Ivask, M., S. Pajusalu, E. Reimann, and S. Koks. 2018. Hippocampus and hypothalamus RNA-sequencing of WFS1-deficient mice. Neuroscience 374: 91–103.
Reimets, R., S. Raud, M. Loomets, T. Visnapuu, V. Volke, A. Reimets, M. Plaas, and E. Vasar. 2016. Variability in the effect of antidepressants upon Wfs1-deficient mice is dependent on the drugs’ mechanism of actions. Behavioural Brain Research 308: 53–63.
Panfili, E., G. Mondanelli, C. Orabona, M.L. Belladonna, M. Gargaro, F. Fallarino, E. Orecchini, P. Prontera, E. Proietti, G. Frontino, E. Tirelli, A. Iacono, C. Vacca, P. Puccetti, U. Grohmann, S. Esposito, and M.T. Pallotta. 2021. Novel mutations in the WFS1 gene are associated with Wolfram syndrome and systemic inflammation. Human Molecular Genetics 30: 265–276.
Jones-Bolin, S., 2012. Guidelines for the care and use of laboratory animals in biomedical research. Current Protocols in Pharmacology 4:Appendix 4B.
Willner, P., A. Towell, D. Sampson, S. Sophokleous, and R. Muscat. 1987. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 93: 358–364.
Hennessy, M.B., T. Deak, and P.A. Schiml-Webb. 2001. Stress-induced sickness behaviors: An alternative hypothesis for responses during maternal separation. Developmental Psychobiology 39: 76–83.
Nolan, M., E. Roman, A. Nasa, K.J. Levins, E. O’Hanlon, V. O’Keane, and D. William Roddy. 2020. Hippocampal and amygdalar volume changes in major depressive disorder: A targeted review and focus on stress. Chronic Stress (Thousand Oaks) 4: 2470547020944553.
Campus, P., V. Colelli, C. Orsini, D. Sarra, and S. Cabib. 2015. Evidence for the involvement of extinction-associated inhibitory learning in the forced swimming test. Behavioural Brain Research 278: 348–355.
Seibenhener M.L. and M.C. Wooten. 2015. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. Journal of Visualized Experiments e52434.
Livak, K.J., and T.D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408.
Ferrua C.P., R. Giorgi, L.C. da Rosa, C.C. do Amaral, G.C. Ghisleni, R.T. Pinheiro and F. Nedel. 2019. MicroRNAs expressed in depression and their associated pathways: a systematic review and a bioinformatics analysis. Journal of Chemical Neuroanatomy 100:101650.
Beurel, E., M. Toups, and C.B. Nemeroff. 2020. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 107: 234–256.
Arioz, B.I., B. Tastan, E. Tarakcioglu, K.U. Tufekci, M. Olcum, N. Ersoy, A. Bagriyanik, K. Genc, and S. Genc. 2019. Melatonin attenuates LPS-induced acute depressive-like behaviors and microglial NLRP3 inflammasome activation through the SIRT1/Nrf2 pathway. Frontiers in Immunology 10: 1511.
Li, W., T. Ali, K. He, Z. Liu, F.A. Shah, Q. Ren, Y. Liu, A. Jiang, and S. Li. 2021. Ibrutinib alleviates LPS-induced neuroinflammation and synaptic defects in a mouse model of depression. Brain, Behavior, and Immunity 92: 10–24.
Ali, T., Q. Hao, N. Ullah, S.U. Rahman, F.A. Shah, K. He, C. Zheng, W. Li, I. Murtaza, Y. Li, Y. Jiang, Z. Tan, and S. Li. 2020. Melatonin act as an antidepressant via attenuation of neuroinflammation by targeting Sirt1/Nrf2/HO-1 signaling. Frontiers in Molecular Neuroscience 13: 96.
Adzic, M., Z. Brkic, M. Mitic, E. Francija, M.J. Jovicic, J. Radulovic, and N.P. Maric. 2018. Therapeutic strategies for treatment of inflammation-related depression. Current Neuropharmacology 16: 176–209.
Aleem, D., and H. Tohid. 2018. Pro-inflammatory cytokines, biomarkers, genetics and the immune system: A mechanistic approach of depression and psoriasis. Rev Colomb Psiquiatr (Engl Ed) 47: 177–186.
Ghosh S., S. Choudhury, O. Chowdhury, S. Mukherjee, A. Das, A. Sain, P. Gupta, A. Adhikary and S. Chattopadhyay. 2020. Inflammation-induced behavioral changes is driven by alterations in Nrf2-dependent apoptosis and autophagy in mouse hippocampus: Role of fluoxetine. Cell Signal 68:109521.
Naeem, K., L. Tariq Al Kury, F. Nasar, A. Alattar, R. Alshaman, F.A. Shah, A.U. Khan, and S. Li. 2021. Natural dietary supplement, carvacrol, alleviates LPS-induced oxidative stress, neurodegeneration, and depressive-like behaviors via the Nrf2/HO-1 pathway. Journal of Inflammation Research 14: 1313–1329.
Yao, W., J.C. Zhang, T. Ishima, C. Dong, C. Yang, Q. Ren, M. Ma, M. Han, J. Wu, H. Suganuma, Y. Ushida, M. Yamamoto, and K. Hashimoto. 2016. Role of Keap1-Nrf2 signaling in depression and dietary intake of glucoraphanin confers stress resilience in mice. Science and Reports 6: 30659.
Giuliani A., S. Gaetani, G. Sorgentoni, S. Agarbati, M. Laggetta, G. Matacchione, M. Gobbi, T. Rossi, R. Galeazzi, G. Piccinini, G. Pelliccioni, A.R. Bonfigli, A.D. Procopio, M.C. Albertini, J. Sabbatinelli, F. Olivieri and F. Fazioli. 2021. Circulating Inflamma-miRs as potential biomarkers of cognitive impairment in patients affected by Alzheimer's disease. Frontiers in Aging Neuroscience 13: 647015.
Tan, L., L. Liu, Z. Jiang, and X. Hao. 2019. Inhibition of microRNA-17-5p reduces the inflammation and lipid accumulation, and up-regulates ATP-binding cassette transporterA1 in atherosclerosis. Journal of Pharmacological Sciences 139: 280–288.
Sequeira, A., C. Kim, M. Seguin, A. Lesage, N. Chawky, A. Desautels, M. Tousignant, C. Vanier, O. Lipp, C. Benkelfat, G. Rouleau, and G. Turecki. 2003. Wolfram syndrome and suicide: Evidence for a role of WFS1 in suicidal and impulsive behavior. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics 119B: 108–113.
Kato, T., M. Ishiwata, K. Yamada, T. Kasahara, C. Kakiuchi, K. Iwamoto, K. Kawamura, H. Ishihara, and Y. Oka. 2008. Behavioral and gene expression analyses of Wfs1 knockout mice as a possible animal model of mood disorder. Neuroscience Research 61: 143–158.
Visnapuu, T., S. Raud, M. Loomets, R. Reimets, S. Sutt, H. Luuk, M. Plaas, S. Koks, V. Volke, A. Alttoa, J. Harro, and E. Vasar. 2013. Wfs1-deficient mice display altered function of serotonergic system and increased behavioral response to antidepressants. Frontiers in Neuroscience 7: 132.
Author information
Authors and Affiliations
Contributions
X. H—conceptualization, methodology, software. Q. Q—data curation, writing (original draft preparation). D. R—writing (reviewing and editing).
Corresponding author
Ethics declarations
Research Involving Human Participants and/or Animals
The animal experiment followed the Guidelines for the Use and Management of Laboratory Animals and was approved by the Laboratory Animal Ethics Committee of Wenzhou Medical University.
Informed Consent
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, X., Qu, Q. & Ren, D. Nrf2 Alleviates Cognitive Dysfunction and Brain Inflammatory Injury via Mediating Wfs1 in Rats with Depression-Like Behaviors. Inflammation 45, 399–413 (2022). https://doi.org/10.1007/s10753-021-01554-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01554-4