Abstract
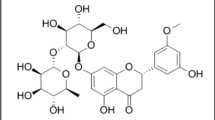
The purpose of the current investigation is to standardize the aqueous leaf extract of Coriandrum sativum L. with a high-performance thin-layer chromatography (HPTLC) method. The method involves the determination and validation of extract with quercetin as standard. Chromatography was performed on pre-coated silica gel G 60 F254 plate as the stationary phase with formic acid, ethyl acetate and toluene as the mobile phase in the ratio 1:4:5 (V/V). Identification and quantification were conducted optical densitometrically at 254 nm. The RF value of quercetin obtained for the standard as well as the leaf extract was found to be 0.50. The method was validated for intra-day and inter-day precision and accuracy. The accuracy of the method was established by a recovery study, with 98.04 ± 0.21% average recovery of quercetin. The lyophilized coriander extract samples contained 0.058 ± 0.02% quercetin. The HPTLC method resulted in the development of an accurate, precise, reproducible and pragmatic approach to standardize the aqueous leaf extract of C. sativum L.





Similar content being viewed by others
References
Ishikawa T, Kondo K, Kitajima J (2003) Water-soluble constituents of coriander. Chem Pharm Bull 51:32–39
Al-Snafi AE (2016) A review on chemical constituents and pharmacological activities of Coriandrum sativum. IOSR J Pharm 6:17–42
Pathak NL, Kasture SB, Bhatt NM, Rathod JD (2011) Phytopharmacological properties of Coriandrum sativum as a potential medicinal tree: an overview. J App Pharm Sci 1:20–25
Rajeshwari U, Andallu B (2011) Medicinal benefits of coriander (Coriandrum sativum L.). Spatula DD 1:51–58
Rathabai V, Kanimozhi D (2012) Evaluation of anti-microbial activity of Coriandrum sativum. Int J Sci Res 3:01–10
Emamghoreishi M, Heidari-Hamedani G (2006) Sedative-hypnotic activity of extracts and essential oil of coriander seeds. Iran J Med Sci 31:22–27
Aissaoui A, Zizi S, Israili ZH, Lyoussi B (2011) Hypoglycemic and Hypolipidemic effects of Coriandrum sativum L. in Merionesshawi rats. J Ethnopharmacol 137:652–661
Asgarpanah J, Kazemivash N (2012) Phytochemistry, pharmacology and medicinal properties of Coriandrum sativum L. Afr J Pharm Pharmaco 6:2340–2345
Liu QF, Jeong H, Lee JH, Hong YK, Oh Y, Kim YM, Suh YS, Bang S, Yun HS, K. Lee K, Cho SM, Lee SB, Jeon S, Chin YW, Koo BS, Cho KS, (2016) Coriandrum sativum Suppresses Aβ42-Induced ROS Increases, Glial Cell Proliferation, and ERK Activation. Am J Chin Med 44:1325–1347
Hashim M, Lincy S, Remya V, Teena M, Anila L (2005) Effect of polyphenolic compounds from Coriandrum sativum on H2O2-induced oxidative stress in human lymphocytes. Food Chem 92:653–660
Nambiar VS, Daniel M, Guin P (2010) Characterization of polyphenols from coriander leaf (Coriandrum sativum), red amaranthus (A. paniculatus) and green amaranthus (A. frumentaceus) using paper chromatography and their health implications. J Herb Med Toxicol 4:173–177
Saranya S, Eswari A, Gayathri E, Eswari S, Vijayarani K (2017) Green synthesis of metallic nanoparticles using aqueous plant extract and their antibacterial activity. Int J Curr Microbiol Appl Sci 6:1834–1845
Iravani S (2011) Green synthesis of metal nanoparticles using plants. Green Chem 13:2638–2650
Bhat S, Kaushal P, Kaur M, Sharma HK (2014) Coriander (Coriandrum sativum L.): processing, nutritional and functional aspects. Afr J Plant Sci 8:25–33
Mukherjee D, Kumar NS, Khatua T, Mukherjee PK (2010) Rapid validated HPTLC method for estimation of betulinic acid in Nelumbo nucifera (Nymphaeaceae) rhizome extract. Phytochem Anal 21:556–560
Ilbeigi V, Tabrizchi M (2015) Thin layer chromatography-ion mobility spectrometry (TLC-IMS). Anal Chem 87:464–469
Wang T, Jin X, Chen Z, Megharaj M, Naidu R (2014) Green synthesis of Fe nano particles using eucalyptus leaf extracts for treatment of eutrophic wastewater. Sci Total Environ 466–467:210–213
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200
Robak J, Gryglewski RJ (1988) Flavonoids are scavengers of super-oxides anions. Biochem Pharmacol 37:837–841
Pick E, Mizel D (1981) Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods 46:211–226
Aruoma OI (1999) Free radicals, antioxidants and international nutrition. Asia Pac J Clin Nut 8:53-e63
Sethi PD (1996) HPTLC, 1st edn. CBS publishers and distributors, New Delhi, p 39
ICH Harmonised Tripartite Guideline (2005) Validation of analytical procedures: text and Methodology, Q2 (R1) Geneva
Ferenczi-Fodor K, Renger B, Vegh Z (2010) The frustrated reviewer—recurrent failures in manuscripts describing validation of quantitative TLC/HPTLC procedures for analysis of pharmaceuticals. J Planar Chromatogr 23:173–179
Ferenczi-Fodor K, Nagy-Turák A (1995) Validation and monitoring of quantitative thin layer chromatographic purity tests for bulk drug substances. J Planar Chromatogr 8:349–356
Acknowledgements
We are grateful to the Department of Pharmaceutical Sciences and Drug Research, Punjabi University, Patiala, Punjab, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Conflict of interest
The authors have no known conflicts of interest associated with this article.
Rights and permissions
About this article
Cite this article
Singh, K., Chopra, D.S., Singh, D. et al. Development of a validated high-performance thin-layer chromatography method for standardization of aqueous extract from the leaf of Coriandrum sativum L.. JPC-J Planar Chromat 34, 337–343 (2021). https://doi.org/10.1007/s00764-021-00121-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00764-021-00121-9