Abstract
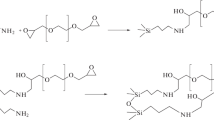
New stationary phases for hydrophilic chromatography (HILIC) with functional layers formed by the multicomponent Ugi reaction have been obtained. Acetone, glycolic acid, ethyl isocyanacetate, and 3‑aminopropyl silica, which also act as adsorbent matrices, were used as reaction components. The study of the effect of solvent on the reaction yield showed that a high degree of coverage of the matrix was achieved when the reaction was carried out in ethanol. The new adsorbents’ chromatographic properties compared with the initial matrix were assessed using the Tanaka tests for hydrophilic phases and by studying the retention of polar analytes from various classes. The synthesized adsorbents have demonstrated high efficiency and selectivity in separating model mixtures of sugars, amino acids, and water-soluble vitamins in the HILIC mode.






Similar content being viewed by others
REFERENCES
Buszewski, B. and Noga, S., Anal. Bioanal. Chem., 2012, vol. 402, p. 231.
Chernobrovkina, A.V., Smolenkov, A.D., and Shpigun, O.A., Lab. Proizvod., vol. 4, no. 4, p.76.
Moni, L., Ciogli, A., Acquarica, I., Dondoni, A., Gasparrini, F., and Marra, A., Chem.-Eur. J., 2010, vol. 16, p. 5712.
Guo, Z., Jin, Y., Liang, T., Liu, Y., Xu, Q., Liang, X., and Lei, A., J. Chromatogr. A, 2009, vol. 1216, p. 257.
Shen, A., Guo, Z., Yu, L., Cao, L., and Liang, X., Chem. Commun., 2011, vol. 47, p. 4550.
Gargano, A.F.G., Leek, T., Lindner, W., and Lammerhofer, M., J. Chromatogr. A, 2013, vol. 1317, p. 12.
de Castro Ferreira, C., Gama, M.R., Silva, G.S., Pereira Almeida, W., Hollingworth Collins, C., and Sales Fontes Jardim, I.C., J. Sep. Sci., 2018, vol. 41, p. 3855.
Kotoni, D., d’Acquarica, I., Ciogli, A., Villani, C., Capitani, D., and Gasparrini, F., J. Chromatogr. A, 2012, vol. 1232, p. 196.
Ugi, I. and Domling, A., Angew. Chem., Int. Ed. Engl., 2000, vol. 39, p. 3168.
Mironov, M.A. and Babaev, E.V., Ross. Khim. Zh., 2009, vol. 53, no. 5, p. 139.
Krasavin, M., Parchinsky, V., Shumsky, A., Konstantinov, I., and Vantskul, A., Tetrahedron Lett., 2010, vol. 51, p. 1367.
Strocker, A., Keating, T., Tempest, P., and Armstrong, R., Tetrahedron Lett., 1996, vol. 37, p. 1149.
Hoppe, D. and Schollkopf, U., Liebigs Ann. Chem., 1972, vol. 763, p. 1183.
Tietze, L.F. and Eicher, T., Reaktionen und Synthesen im organisch-chemischen Praktikum und Forschungslaboratorium, Weinheim: Wiley, 1991.
Dolci, M., Chromatographic Characterization of Stationary Phases for Hydrophilic Interaction Liquid Chromatography, Runcorn, UK: Thermo Fisher Sci., 2013.
Kawachi, Y., Ikegami, T., Takubo, H., Ikegami, Y., Miyamoto, M., and Tanaka, N., J. Chromatogr. A, 2011, vol. 1218, p. 5903.
Popov, A.S., Maksimov, G.S., Smolenkov, A.D., Shpigun, O.A., and Chernobrovkina, A.V., Moscow Univ. Chem Bull. (Engl. Transl.), 2021, vol. 76, no. 2, p. 75.
Fu, Q., Liang, T., Li, Z., Xu, X., Ke, Y., Jin, Y., and Liang, X., Carbohydr. Res., 2013, vol. 379, p. 13.
Jenkins, K., J. Chromatogr. Today, 2015, vol. 8, p. 14.
Themelis, T., Gotti, R., and Gatti, R., J. Pharm. Biomed. Anal., 2017, vol. 145, p. 751.
Karatapanis, A.E., Fiamegos, Y.C., and Stalikas, C.D., J. Sep. Sci., 2009, vol. 32, p. 909.
Sarvin, B.A., Seregin, A.P., Shpigun, O.A., Rodin, I.A., and Stavrianidi, A.N., J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2018, vol. 1092, p. 138.
Funding
The work is supported by the Russian Science Foundation, project no. 20-13-00140.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Zhukova
Rights and permissions
About this article
Cite this article
Chikurova, N.Y., Shemyakina, A.O., Bryskina, D.E. et al. A Novel Adsorbent for Hydrophilic Chromatography Based on Silica Modified by the Ugi Reaction. J Anal Chem 76, 1083–1092 (2021). https://doi.org/10.1134/S1061934821090033
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934821090033