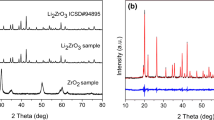
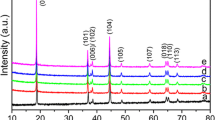
Abstract
Graphite is considered as an ideal anode material for lithium-ion battery (LIB) due to its high stability, good conductivity and wide source of availability. However, the low energy density and theoretical capacity of graphite cannot meet the needs of high performance anode materials. To circumvent this issue, alternative materials have been sought for many years now. Herein, we report the synthesis of highly crystalline lithium iron molybdate LiFe(MoO4)2 by combustion method and evaluated its performance as an anode material for lithium-ion batteries. Triclinic LiFe(MoO4)2 crystals having particle size 2–5 μm with good crystallinity were obtained. The material shows long cycle life and high rate performance than commercial graphite and exhibits first reversible discharge capacity of 931.6 mAh/g at a current density of 100 mA/g which is three times higher than commercial graphite. The high specific capacity together with the outstanding rate and cycle performance makes LiFe(MoO4)2 a promising anode material for LIB. A detailed analysis on the crystal structure and electronic properties of LiFe(MoO4)2 is presented based on DFT studies to complement the experimental observations.











Similar content being viewed by others
References
M. Han, Y. Mu, J. Yu, Nanoscopically and uniformly distributed SnO2@TiO2/C composite with highly mesoporous structure and bichemical bonds for enhanced lithium ion storage performances. Mater. Adv. 1, 421–429 (2020)
K. Hosoya, T. Kamidaira, T. Tsuda, A. Imanishi, M. Haruta, T. Doi, M. Inaba, S. Kuwabata, Lithium-ion battery performance enhanced by the combination of Si thin flake anodes and binary ionic liquid systems. Mater. Adv. 1, 625–631 (2020)
M.S. Tamboli, H.S. Jadhav, D.R. Patil, A.F. Shaikh, S.S. Patil, J.G. Seo, H. Choi, S.W. Gosavi, B. B. Kale, Hierarchical novel NiCo2O4/BiVO4 hybrid heterostructure as an advanced anode material for rechargeable lithium ion battery. Int. J. Energy Res. 44, 12126–12135 (2020)
M. Winter, J.O. Besenhard, M.E. Spahr, P. Novák, Insertion electrode materials for rechargeable lithium batteries. J. Adv. Mater. 10(10), 725–763 (1998)
G.-A. Nazri, G. Pistoia, Lithium Batteries: Science and Technology (Springer Science + Business Media, New York, USA, 2008)
K. Zhong, X. Xia, B. Zhang, H. Li, Z. Wang, L. Chen, MnO powder as anode active materials for lithium ion batteries. J. Power Sources 195, 3300–3308 (2010)
D. Xu, G. Luo, J. Yu, W. Chen, C. Zhang, D. Ouyang, Y. Fang, X. Yu, Quadrangular-CNT-Fe3O4-C composite based on quadrilateral carbon nanotubes as anode materials for high performance lithium-ion batteries. J. Alloys Compd. 702, 499–508 (2017)
W. Xie, L. Gu, F. Xia, B. Liu, X. Hou, Q. Wang, D. Liu, D. He, Fabrication of voids-involved SnO2@C nanofibers electrodes with highly reversible Sn/SnO2 conversion and much enhanced coulombic efficiency for lithium-ion batteries. J. Power Sources 327, 21–28 (2016)
D.T. Ngo, R.S. Kalubarme, H.T.T. Le, C.-N. Park, C.-J. Park, Conducting additive-free amorphous GeO2/C composite as a high capacity and long-term stability anode for lithium ion batteries. Nanoscale 7, 2552–2560 (2015)
M.V. Reddy, Y. Xu, V. Rajarajan, T. Ouyang, B.V.R. Chowdari, Template free facile molten synthesis and energy storage studies on MCo2O4 (M = Mg, Mn) as anode for Li-ion batteries. ACS Sustain. Chem. Eng. 3, 3035–3042 (2015)
A.F. Shaikh, R.S. Kalubarme, M.S. Tamboli, S.S. Patil, M.V. Kulkarni, D.R. Patil, S.W. Gosavi, C.-J. Park, B.B. Kale, Nanowires of Ni substituted MnCo2O4 as an anode material for high performance lithium-ion battery. ChemistrySelect 2, 4630–4637 (2017)
M.V. Reddy, Z. Beichen, K.P. Loh, B.V.R. Chowdari, Facile synthesis of Co3O4 by molten salt method and its Li-storage performance. CrystEngComm 15, 3568–3574 (2013)
L. Wang, Y. Zheng, X. Wang, S. Chen, F. Xu, L. Zuo, J. Wu, L. Sun, Z. Li, H. Hou, Y. Song, Nitrogen-doped porous carbon/Co3O4 nanocomposites as anode materials for lithium-ion batteries. ACS Appl. Mater. Interfaces 6(10), 7117–7125 (2014)
G. Huang, S. Xu, S. Lu, L. Li, H. Sun, Micro-/nanostructured Co3O4 anode with enhanced rate capability for lithium-ion batteries. ACS Appl. Mater. Interfaces 6, 7117–7125 (2014)
Z.W. Zhao, Z.P. Guo, H.K. Liu, Non-aqueous synthesis of crystalline Co3O4 powders using alcohol and cobalt chloride as a versatile reaction system for controllable morphology. J. Power Sources 147(1–2), 264–268 (2005)
L. Li, K.H. Seng, Z. Chen, Z. Guo, H.K. Liu, Self-assembly of hierarchical star-like Co3O4 micro/nanostructures and their application in lithium ion batteries. Nanoscale 5(5), 1922–1928 (2013)
A.M. Hashem, A.E. Abdel-Ghany, R.S. El-Tawil, S. Indris, H. Ehrenberg, A. Mauger, C.M. Julien, Amorphous Mo5O14-type/carbon nanocomposite with enhanced electrochemical capability for lithium-ion batteries. J. Nanomater. 10(1), 8 (2020)
A. Manthiram, J.B. Goodenough, Lithium insertion into Fe2(SO4)3 frameworks. J. Power Sources 26, 403–408 (1989)
C. Masquelier, A.K. Padhi, K.S. Nanjundaswamy, J.B. Goodenough, New cathode materials for rechargeable lithium batteries: the 3-D framework structures Li3Fe2(XO4)3 (X = P, As). J. Solid State Chem. 135(2), 228–234 (1998)
K.S. Nanjundaswamy, A.K. Padhi, J.B. Goodenough, S. Okada, H. Ohtsuka, H. Arai, J. Yamaki, Synthesis, redox potential evaluation and electrochemical characteristics of NASICON-related-3D framework compounds. Solid State Ion. 92, 1–0 (1996)
M. Alvarez-Vega, U. Amador, M.E.A. Dompablo, Electrochemical study of Li3Fe(MoO4)3 as positive electrode in lithium cells. J. Electrochem. Soc. 152, A1306 (2005)
S. Chen, H. Duan, Z. Zhou, Q. Fan, Y. Zhao, Y. Dong, Lithiated bimetallic oxide, Li3Fe(MoO4)3, as a high-performance anode material for lithium-ion batteries and its multielectron reaction mechanism. J. Power Sources 476, 228656 (2020)
C. Wurm, M. Morcrette, G. Rousse, L.c. Dupont, C. Masquelier, Lithium insertion/extraction into/from LiMX2O7 compositions (M = Fe, V; X = P, As) prepared via a solution method. Chem. Mater. 14, 2701–2710 (2002)
A.M. Tamboli, M.S. Tamboli, C.S. Praveen, P.K. Dwivedi, I. Karbhal, S.W. Gosavi, M.V. Shelke, B.B. Kale, Architecture of NaFe(MoO4)2 as a novel anode material for rechargeable lithium and sodium ion batteries. Appl. Surf. Sci. 559, 149903 (2021)
A.K. Padhi, K.S. Nanjundaswamy, J.B. Goodenough, Phospho-olivines as positive‐electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 144, 1188 (1997)
M. Anji Reddy, V. Pralong, V. Caignaert, U.V. Varadaraju, B. Raveau, Monoclinic iron hydroxy sulphate: a new route to electrode materials. Electrochem. Commun. 11, 1807–1810 (2009)
S.-H. Lee, Y.-H. Kim, R. Deshpande, P.A. Parilla, E. Whitney, D.T. Gillaspie, K.M. Jones, A.H. Mahan, S. Zhang, A.C. Dillon, Reversible lithium-ion insertion in molybdenum oxide nanoparticles. J. Adv. Mater. 20, 3627–3632 (2008)
H. Yu, C. Guan, X. Rui, B. Ouyang, B. Yadian, Y. Huang, H. Zhang, H.E. Hoster, H.J. Fan, Q. Yan, Hierarchically porous three-dimensional electrodes of CoMoO4 and ZnCo2O4 and their high anode performance for lithium ion batteries. Nanoscale 6, 10556–10561 (2014)
Z. Zhang, W. Li, T.-W. Ng, W. Kang, C.-S. Lee, W. Zhang, Iron (ii) molybdate (FeMoO4) nanorods as a high-performance anode for lithium ion batteries: structural and chemical evolution upon cycling. J. Mater. Chem. A 3, 20527–20534 (2015)
L. Wang, Y. He, Y. Mu, M. Liu, Y. Chen, Y. Zhao, X. Lai, J. Bi, D. Gao, Sintering temperature induced evolution of microstructures and enhanced electrochemical performances: sol-gel derived LiFe(MoO4)2 microcrystals as a promising anode material for lithium-ion batteries. Front. Chem. 6, 492 (2018)
L. Wang, Y. He, Y. Mu, B. Wu, M. Liu, Y. Zhao, X. Lai, J. Bi, D. Gao, Sol–gel driving LiFe(MoO4)2 microcrystals: high capacity and superior cycling stability for anode material in lithium ion batteries. Electron. Mater. Lett. 15, 186–191 (2019)
M. Devi, U.V. Varadaraju, Lithium insertion in lithium iron molybdate. Electrochem. Commun. 18, 112–115 (2012)
N. Chen, Y. Yao, D. Wang, Y. Wei, X. Bie, C. Wang, G. Chen, F. Du, LiFe(MoO4)2 as a novel anode material for lithium-ion batteries. ACS Appl. Mater. Interfaces 6, 10661–10666 (2014)
H. Kockar, E. Ozergin, O. Karaagac, M. Alper, Characterisations of CoFeCu films: influence of Fe concentration. J. Alloys Compd. 586, S326–S330 (2014)
A. Tekgül, M. Alper, H. Kockar, M. Haciismailoglu, The effect of ferromagnetic and non-ferromagnetic layer thicknesses on the electrodeposited CoFe/Cu multilayers. J. Mater. Sci. Mater. Electron. 26(4), 2411–2417 (2015)
G. Kresse, J. Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6(1), 15–50 (1996)
G. Kresse, J. Hafner, Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47(1), 558 (1993)
G. Kresse, J. Hafner, Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 49(20), 14251 (1994)
P.E. Blöchl, Projector augmented-wave method. Phys. Rev. B 50(24), 17953 (1994)
J.P. Perdew, J.A. Chevary, S.H. Vosko, K.A. Jackson, M.R. Pederson, D.J. Singh, C. Fiolhais, Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 46(11), 6671 (1992)
J.P. Perdew, J.A. Chevary, S.H. Vosko, K.A. Jackson, M.R. Pederson, D.J. Singh, C. Fiolhais, Erratum: Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 48(7), 4978 (1993)
S.L. Dudarev, G.A. Botton, S.Y. Savrasov, C.J. Humphreys, A.P. Sutton, Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+ U study. Phys. Rev. B 57(3), 1505 (1998)
M. Liu, Y. Zhang, T. Zou, V.O. Garlea, T. Charlton, Y. Wang, F. Liu, Y. Xie, X. Li, L. Yang, B. Li, Antiferromagnetism of double molybdate LiFe(MoO4)2. Inorg. Chem. 59(12), 8127–8133 (2020)
H.J. Monkhorst, J.D. Pack, Special points for Brillouin-zone integrations. Phys. Rev. B 13(12), 5188 (1976)
A.V.D. Lee, M. Beaurain, P. Armand, LiFe(MoO4)2, LiGa(MoO4)2 and Li3Ga(MoO4)3. Acta Crystallogr. C 64(1), i1–i4 (2008)
N. Hornsveld, B. Put, W.M.M. Kessels, P.M. Vereecken, M. Creatore, Mass spectrometry study of Li2CO3 film growth by thermal and plasma-assisted atomic layer deposition. J. Phys. Chem. C 123(7), 4109–4115 (2019)
L. Castro, R. Dedryvere, M. El Khalifi, P.E. Lippens, J. Bréger, C. Tessier, D. Gonbeau, The spin-polarized electronic structure of LiFePO4 and FePO4 evidenced by in-lab XPS. J. Phys. Chem. C 114(41), 17995–18000 (2010)
Z. Cao, B. Wei, High rate capability of hydrogen annealed iron oxide–single walled carbon nanotube hybrid films for lithium-ion batteries. ACS Appl. Mater. Interfaces 5(20), 10246–10252 (2013)
Z.-Y. Yu, Y. Duan, M.-R. Gao, C.-C. Lang, Y.-R. Zheng, S.-H. Yu, A one-dimensional porous carbon-supported Ni/Mo2C dual catalyst for efficient water splitting. Chem. Sci. 8(2), 968–973 (2017)
B. Guo, X. Fang, B. Li, Y. Shi, C. Ouyang, Y.-S. Hu, Z. Wang, G.D. Stucky, L. Chen, Synthesis and lithium storage mechanism of ultrafine MoO2 nanorods. Chem. Mater. 24(3), 457–463 (2012)
J.H. Ku, Y.S. Jung, K.T. Lee, C.H. Kim, S.M. Oh, Thermoelectrochemically activated MoO2 powder electrode for lithium secondary batteries. J. Electrochem. Soc. 156(8), A688 (2009)
T. Yoon, C. Chae, Y.-K. Sun, X. Zhao, H.H. Kung, J.K. Lee, Bottom-up in situ formation of Fe3O4 nanocrystals in a porous carbon foam for lithium-ion battery anodes. J. Mater. Chem. 21(43), 17325–17330 (2011)
W. Li, F. Cheng, Z. Tao, J. Chen, Vapor-transportation preparation and reversible lithium intercalation/deintercalation of α-MoO3 microrods. J. Phys. Chem. B 110(1), 119–124 (2006)
D. Aurbach, Y. Talyosef, B. Markovsky, E. Markevich, E. Zinigrad, L. Asraf, J.S. Gnanaraj, H.J. Kim, Design of electrolyte solutions for Li and Li-ion batteries: a review. Electrochim. Acta 50, 247–254 (2004)
J. Christensen, J.A. Newman, Mathematical model for the lithium-ion negative electrode solid electrolyte interphase. J. Electrochem. Soc. 151, A1977–A1988 (2004)
S. Gantenbein, M. Schönleber, M. Weiss, E. Ivers-Tiffee, Capacity fade in lithium-ion batteries and cyclic aging over various state-of-charge ranges. Sustainability 11(23), 6697 (2019)
G. Zhou, D.-W. Wang, F. Li, L. Zhang, N. Li, Z.-S. Wu, L. Wen, G.Q. Lu, H.-M. Cheng, Graphene-wrapped Fe3O4 anode material with improved reversible capacity and cyclic stability for lithium ion batteries. Chem. Mater. 22(18), 5306–5313 (2010)
T. Muraliganth, A. Vadivel Murugan, A. Manthiram, Facile synthesis of carbon-decorated single-crystalline Fe3O4 nanowires and their application as high performance anode in lithium ion batteries. Chem. Commun. 47, 7360–7362 (2009)
L. Liu, M. An, P. Yang, J. Zhang, Superior cycle performance and high reversible capacity of SnO2/graphene composite as an anode material for lithium-ion batteries. Sci. Rep. 5(1), 1–0 (2015)
Acknowledgements
Dr. Asiya M. Tamboli (Asiya F. Shaikh) acknowledges CSIR, New Delhi for financial support with Award Number 09/882(0008)/2K13-EMR-I. Dr. C. S. Praveen would like to thank DST-India for INSPIRE Faculty Fellowship (Award Number IFA-18 PH217). Dr. C. S. Praveen also acknowledges Dr. Aleix-Comas Vives for his support with the VASP Calculations. Authors are grateful to the Ministry of Electronics and Information Technology (MeitY), New Delhi and Nanocrystalline Material and Glass Laboratory Group, C-MET, Pune. This work was supported by “Human Resources Program in Energy Technology” of the Korea Institute of Energy Technology Evaluation and Planning (KETEP), granted financial resource from the Ministry of Trade, Industry & Energy, Republic of Korea (No. 20204010600100).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tamboli, A.M., Tamboli, M.S., Dwivedi, P.K. et al. Engineering microstructure of LiFe(MoO4)2 as an advanced anode material for rechargeable lithium-ion battery. J Mater Sci: Mater Electron 32, 24273–24284 (2021). https://doi.org/10.1007/s10854-021-06892-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-06892-5