Abstract
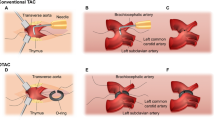
Transverse aortic constriction (TAC) in mice is the most popular model to mimic pressure overload heart disease. In this study, we developed a convenient, quick, and less invasive new TAC mice model. Briefly, after anesthetization, endotracheal intubation was then performed, and the endotracheal tube was connected to a ventilator. The second intercostal space was opened and then the home-made retractors were used to push aside the thymus gently. A tunnel under the aortic arch was made and a segment of 6–0 monofilament polypropylene suture which had been threaded through a specifically modified blunted 26-gauge syringe needle was passed through the tunnel. A blunted 27-gauge needle was placed parallel to the transverse aorta and then three knots were tied quickly. After ligation, the spacer was removed promptly and gently to achieve a constriction of 0.4 mm in diameter. Five weeks after TAC, cardiac hypertrophy, fibrosis, and left ventricular dysfunction were observed.
Graphical abstract
The mouse was anesthetized with pentobarbital (50 mg/kg) via intraperitoneal injection. Endotracheal intubation under direct vision was then performed and the endotracheal tube was connected to a ventilator. The second intercostal space was opened and then the home-made retractors were used to push aside the thymus gently. A tunnel under the aortic arch was made and a segment of 6–0 monofilament polypropylene suture which had been threaded through a specifically modified blunted 26-gauge syringe needle was passed through the tunnel.








Similar content being viewed by others
Abbreviations
- TAC:
-
Transverse aortic constriction
- LV:
-
Left ventricular
- BCA:
-
Brachiocephalic artery
- LCCA:
-
Left common carotid artery
- LVIDd:
-
LV end-diastolic internal diameter
- LVIDs:
-
LV end-systolic internal diameter
- IVSd:
-
Interventricular septum end-diastolic thickness
- IVSs:
-
Interventricular septum end-systolic thickness
- LVPWd:
-
LV end-diastolic posterior wall thickness
- LVPWs:
-
LV end-systolic posterior wall thickness
- LVEDV:
-
LV end-diastolic volume
- LVESV:
-
LV end-systolic volume
- LVEF:
-
LV ejection fraction
- LVFS:
-
LV fractional shortening
- HW:
-
Heart weight
- BW:
-
Body weight
- TL:
-
Tibia length
- H&E:
-
Hematoxylin-eosin
- MT:
-
Masson’s trichrome
- AAo:
-
Ascending aorta
References
Shimizu, I., & Minamino, T. (2016). Physiological and pathological cardiac hypertrophy. Journal of Molecular and Cellular Cardiology, 97, 245–262. https://doi.org/10.1016/j.yjmcc.2016.06.001
Cuspidi, C., Sala, C., Negri, F., Mancia, G., & Morganti, A. (2012). Prevalence of left-ventricular hypertrophy in hypertension: An updated review of echocardiographic studies. Journal of Human Hypertension, 26(6), 343–349. https://doi.org/10.1038/jhh.2011.104
Lackland, D. T., & Weber, M. A. (2015). Global burden of cardiovascular disease and stroke: Hypertension at the core. Canadian Journal of Cardiology, 31(5), 569–571. https://doi.org/10.1016/j.cjca.2015.01.009
Mills, K. T., Bundy, J. D., Kelly, T. N., Reed, J. E., Kearney, P. M., Reynolds, K., et al. (2016). Global disparities of hypertension prevalence and control: A systematic analysis of population-based studies from 90 countries. Circulation, 134(6), 441–450. https://doi.org/10.1161/circulationaha.115.018912
Sorrentino, M. J. (2019). The evolution from hypertension to heart failure. Heart Failure Clinics, 15(4), 447–453. https://doi.org/10.1016/j.hfc.2019.06.005
Muiesan, M. L., Salvetti, M., Rizzoni, D., Paini, A., Agabiti-Rosei, C., Aggiusti, C., et al. (2013). Resistant hypertension and target organ damage. Hypertension Research, 36(6), 485–491. https://doi.org/10.1038/hr.2013.30
Zhou, B., Perel, P., Mensah, G. A., & Ezzati, M. (2021). Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nature Reviews. Cardiology. https://doi.org/10.1038/s41569-021-00559-8
Laurent, S. (2017). Antihypertensive drugs. Pharmacological Research, 124, 116–125. https://doi.org/10.1016/j.phrs.2017.07.026
Rockman, H. A., Ross, R. S., Harris, A. N., Knowlton, K. U., Steinhelper, M. E., Field, L. J., et al. (1991). Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proceedings of the National Academy of Sciences of the United States of America, 88(18), 8277–8281. https://doi.org/10.1073/pnas.88.18.8277
Molinari, F., Malara, N., Mollace, V., Rosano, G., & Ferraro, E. (2016). Animal models of cardiac cachexia. International Journal of Cardiology, 219, 105–110. https://doi.org/10.1016/j.ijcard.2016.05.071
Laroumanie, F., Douin-Echinard, V., Pozzo, J., Lairez, O., Tortosa, F., Vinel, C., et al. (2014). CD4+ T cells promote the transition from hypertrophy to heart failure during chronic pressure overload. Circulation, 129(21), 2111–2124. https://doi.org/10.1161/circulationaha.113.007101
deAlmeida, A. C., van Oort, R. J., & Wehrens, X. H. (2010). Transverse aortic constriction in mice. Journal of Visualized Experiments: JoVE, (38). https://doi.org/10.3791/1729
Eichhorn, L., Weisheit, C. K., Gestrich, C., Peukert, K., Duerr, G. D., Ayub, M. A., et al. (2018). A closed-chest model to induce transverse aortic constriction in mice. Journal of Visualized Experiments: JoVE, (134). https://doi.org/10.3791/57397
Tavakoli, R., Nemska, S., Jamshidi, P., Gassmann, M., Frossard, N. (2017). Technique of minimally invasive transverse aortic constriction in mice for induction of left ventricular hypertrophy. Journal of Visualized Experiments: JoVE, (127). https://doi.org/10.3791/56231
Zaw, A. M., Williams, C. M., Law, H. K., Chow, B. K. (2017). Minimally invasive transverse aortic constriction in mice. Journal of Visualized Experiments: JoVE, (121). https://doi.org/10.3791/55293
Hill, J. A., & Olson, E. N. (2008). Cardiac plasticity. New England Journal of Medicine, 358(13), 1370–1380. https://doi.org/10.1056/NEJMra072139
You, J., Wu, J., Zhang, Q., Ye, Y., Wang, S., Huang, J., et al. (2018). Differential cardiac hypertrophy and signaling pathways in pressure versus volume overload. American Journal of Physiology. Heart and Circulatory Physiology, 314(3), H552–H562. https://doi.org/10.1152/ajpheart.00212.2017
Xiang, F. L., Fang, M., & Yutzey, K. E. (2017). Loss of β-catenin in resident cardiac fibroblasts attenuates fibrosis induced by pressure overload in mice. Nature Communications, 8(1), 712. https://doi.org/10.1038/s41467-017-00840-w
Toischer, K., Rokita, A. G., Unsöld, B., Zhu, W., Kararigas, G., Sossalla, S., et al. (2010). Differential cardiac remodeling in preload versus afterload. Circulation, 122(10), 993–1003. https://doi.org/10.1161/circulationaha.110.943431
Muthuramu, I., Amin, R., Aboumsallem, J. P., Mishra, M., Robinson, E. L., & De Geest, B. (2018). Hepatocyte-specific SR-BI gene transfer corrects cardiac dysfunction in Scarb1-deficient mice and improves pressure overload-induced cardiomyopathy. Arteriosclerosis, Thrombosis, and Vascular Biology, 38(9), 2028–2040. https://doi.org/10.1161/atvbaha.118.310946
Kong, P., Christia, P., & Frangogiannis, N. G. (2014). The pathogenesis of cardiac fibrosis. Cellular and Molecular Life Sciences, 71(4), 549–574. https://doi.org/10.1007/s00018-013-1349-6
López, B., Ravassa, S., Moreno, M. U., José, G. S., Beaumont, J., González, A., et al. (2021). Diffuse myocardial fibrosis: Mechanisms, diagnosis and therapeutic approaches. Nature Reviews. Cardiology. https://doi.org/10.1038/s41569-020-00504-1
Osterholt, M., Nguyen, T. D., Schwarzer, M., & Doenst, T. (2013). Alterations in mitochondrial function in cardiac hypertrophy and heart failure. Heart Failure Reviews, 18(5), 645–656. https://doi.org/10.1007/s10741-012-9346-7
Bakris, G., Ali, W., & Parati, G. (2019). ACC/AHA versus ESC/ESH on hypertension guidelines: JACC guideline comparison. Journal of the American College of Cardiology, 73(23), 3018–3026. https://doi.org/10.1016/j.jacc.2019.03.507
Palmer, S., Albergante, L., Blackburn, C. C., & Newman, T. J. (2018). Thymic involution and rising disease incidence with age. Proceedings of the National Academy of Sciences of the United States of America, 115(8), 1883–1888. https://doi.org/10.1073/pnas.1714478115
Miller, J. (2020). The function of the thymus and its impact on modern medicine. Science, 369(6503), eaba2429.
Dai, X., Hua, L., Chen, Y., Wang, J., Li, J., Wu, F., et al. (2018). Mechanisms in hypertension and target organ damage: Is the role of the thymus key? (Review). International Journal of Molecular Medicine, 42(1), 3–12. https://doi.org/10.3892/ijmm.2018.3605
Miller, J. F., & Osoba, D. (1967). Current concepts of the immunological function of the thymus. Physiological Reviews, 47(3), 437–520. https://doi.org/10.1152/physrev.1967.47.3.437
Frieler, R. A., & Mortensen, R. M. (2015). Immune cell and other noncardiomyocyte regulation of cardiac hypertrophy and remodeling. Circulation, 131(11), 1019–1030. https://doi.org/10.1161/circulationaha.114.008788
Takahashi, M., Kinugawa, S., Takada, S., Kakutani, N., Furihata, T., Sobirin, M. A., et al. (2020). The disruption of invariant natural killer T cells exacerbates cardiac hypertrophy and failure caused by pressure overload in mice. Experimental Physiology, 105(3), 489–501. https://doi.org/10.1113/ep087652
Bosch, L., de Haan, J., Seijkens, T., van Tiel, C., Brans, M., Pasterkamp, G., et al. (2019). Small molecule-mediated inhibition of CD40-TRAF6 reduces adverse cardiac remodelling in pressure overload induced heart failure. International Journal of Cardiology, 279, 141–144. https://doi.org/10.1016/j.ijcard.2018.12.076
Noll, N. A., Lal, H., & Merryman, W. D. (2020). Mouse models of heart failure with preserved or reduced ejection fraction. American Journal of Pathology, 190(8), 1596–1608. https://doi.org/10.1016/j.ajpath.2020.04.006
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 81770319).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Huayang Li, Quan Liu, and Shunjun Wang carried out the experiments. Lin Huang, Yuan Yue, Suiqing Huang, and Kangni Feng helped with the material preparation, data collection, and analysis. The first draft of the manuscript was written by Huayang Li, and all authors commented on previous versions of the manuscript. Zhongkai Wu contributed substantially to the conception, design of the study, acquired funding, supervised the study, and edited the manuscript. All authors read and approved the final manuscript.
Ethics declarations
No human studies were carried out by the authors for this article.
Ethics Approval for Use of Animals
All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the Institutional Animal Use and Care Committee of Sun Yat-sen University (2019001114).
Conflict of Interest
The authors declare no competing interests.
Additional information
Huayang Li and Quan Liu contributed equally to this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
(MP4 521 mb)
Rights and permissions
About this article
Cite this article
Li, H., Liu, Q., Wang, S. et al. A New Minimally Invasive Method of Transverse Aortic Constriction in Mice. J. of Cardiovasc. Trans. Res. 15, 635–643 (2022). https://doi.org/10.1007/s12265-021-10170-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-021-10170-4