Commonly noted postnatal derivatives resulting from incomplete regression of the fetal right valve of the sinus venosus are the Eustachian valve adjacent to the inferior vena cava and the Thebesian valve on the atrial septum bordering the orifice of the coronary sinus. Reference Moral, Ballesteros, Huguet, Panaro, Palet and Evangelista1-Reference Steding, Jinwen, Seidl, Männer and Xia2 The more infrequently seen Chiari network arises from an incomplete fragmented regression with multiple fenestrations and gradual stretching, resulting in a reticulated web of myocardial fibres in the right atrium. This network has been detected at cardiac autopsy in 3.8–4.6% Reference Bhatnagar, Nettleton, Campbell, Wagner, Kuwabara and Muresian3-Reference Klimek-Piotrowska, Hołda, Koziej and Strona5 and visualised on echocardiography in 1.5–4.6% of the population. Reference Werner, Cheitlin, Gross, Speck and Ivey6-Reference Schneider, Hofmann, Justen and Meinertz8 The vast majority are small, confined to the right atrium, and considered to be quite benign, particularly in childhood. The Chiari network is considered unusually prominent when large enough to prolapse through the tricuspid annulus into the right ventricle during diastole Reference Aypar, Sert and Odabaş9 (Fig 1 and Supplementary Videos). While there have been many case reports, to the best of our knowledge, there are no published studies evaluating the impact of a prominent prolapsing Chiari network in a series of patients.
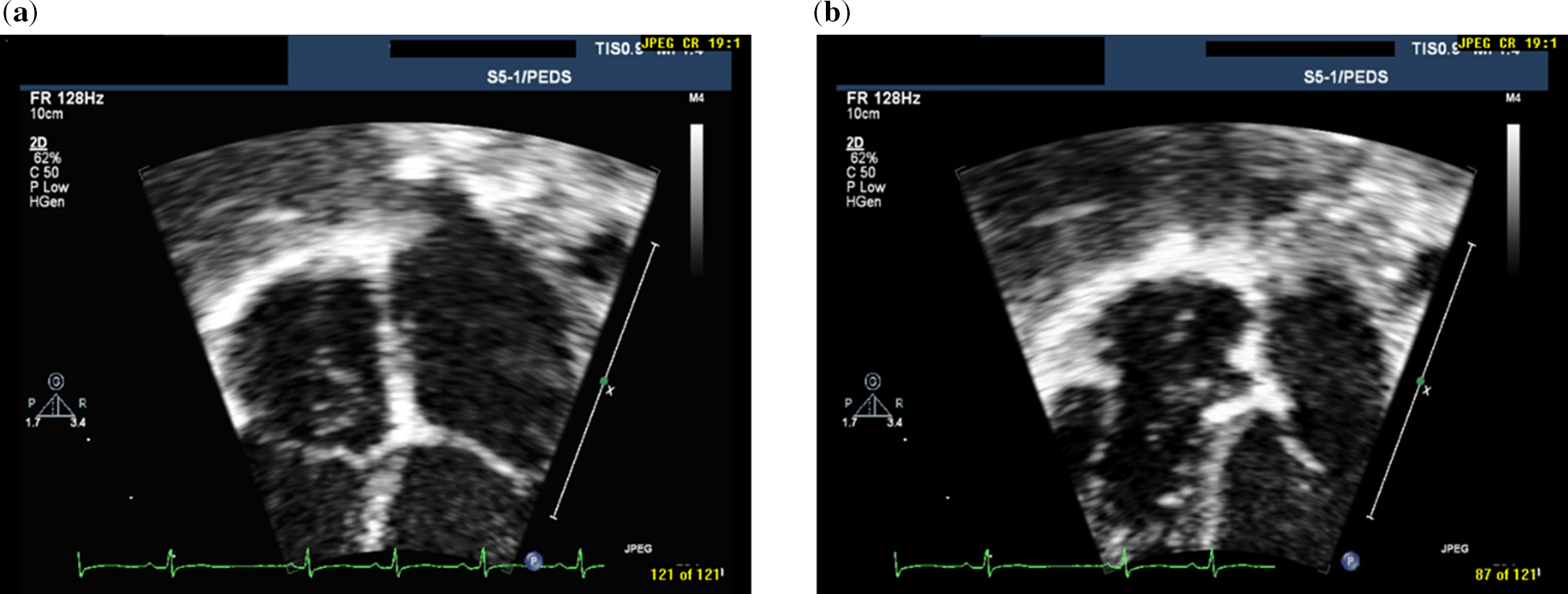
Figure 1. Two-dimensional echocardiographic still frames of prominent prolapsing chiari network in apical four-chamber view during systole ( a) and during diastole (b).
Methods
Study population
Children, 18 years of age or less, who were noted to have a redundant Chiari network prolapsing through the tricuspid annulus during diastole on transthoracic echocardiogram, were identified retrospectively from the echocardiographic database at the Jacksonville Pediatric and Adult Congenital Cardiology outpatient clinic and reviewed. Thirty-eight patients were identified over a period of 12.5 years from March 2008 to November 2020 and evaluated. The presenting reason for cardiology referral, demographics, significant physical exam findings, and pertinent medical history were obtained from the clinical record. Echocardiogram findings were tabulated, along with pertinent results from 12-lead electrocardiograms and any Holter/event monitor studies. Patients who returned for follow-up evaluation had the results of their most recent assessment tabulated for comparison. The study was approved by the Institutional Review Board of the University of Florida-Jacksonville.
Echocardiography
Echocardiograms were obtained using iE33 model echocardiographic instruments (Philips Healthcare, NA) with 1–8 MHz probes following the paediatric protocol recommended by the American Society of Echocardiography. Reference Lopez, Colan and Frommelt10 A prolapsing Chiari network was diagnosed by the presence of mobile filamentous strands in the right atrium with attachments from the Eustachian valve at the orifice of the inferior vena cava across to the lower atrial septum and bowing down into the right ventricle during diastole at least 2 mm below the level of the tricuspid annulus. All echocardiographic measurements were performed off-line in DICOM format using the Philips Xcelera analysis package. Right and left heart echocardiographic measures were obtained according to the paediatric standards of the American Society of Echocardiography. Reference Lopez, Colan and Frommelt10 For the right heart, the maximal inferior vena cava diameter, right atrial area, tricuspid and pulmonic valve annulus diameters, and right ventricular end-diastolic cavity area, and free wall diameter were measured. The degree of tricuspid valve regurgitation was estimated using the vena contracta diameter and its peak instantaneous gradient was measured. Tricuspid regurgitation was graded by the indexed vena contracta diameter Reference Colan and Sleeper11 as trivial if < 1.8 mm/m, mild if 1.8–2.4 mm/m, and moderate if 2.5–3.4 mm/m. For the left heart, the left ventricular end-diastolic septal wall, cavity, and posterior wall diameters, and aortic valve annulus diameter were measured. Echocardiographic z-values were calculated using the methods of Cantinotti et al Reference Cantinotti, Scalese and Murzi12-Reference Cantinotti, Giordano and Scalese13 for right atrial and right ventricular cavity areas and maximal inferior vena cava diameter, and Lopez et al Reference Lopez, Colan and Stylianou14 for all other values. The z-scores were determined using the calculator at parameterz.com.
Statistical analysis
Body surface area was calculated using the formula of Haycock. Reference Haycock, Schwartz and Wisotsky15 Echocardiographic parameters were indexed to body surface area, and when available to z-values, and then compared. Intra-observer variability was analysed for pairs of all measurements in 10 patients each using Spearman correlation and intra-class correlation coefficients. Results are presented as mean ± standard deviation for parameters that were normally distributed and as median with range for non-normal quantitative data. The presence of a normal distribution was determined using the Shapiro–Wilk test. Analysis of variance followed by the Holm–Bonferroni method was used for comparison of results between patient groups. The two-tailed paired Student’s t-test was used within the follow-up patient group for comparison of results between the initial evaluation and the latest follow-up data. Pearson linear regression analysis was used to determine correlation coefficients between parameters. A p-value < 0.05 was considered statistically significant. Statistical analysis was carried out using SPSS Statistics for Windows, Version 26.0, IBM Corp., Armonk, NY.
Results
Study population at presentation
A total of 38 paediatric patients, 21 females and 17 males, were identified with 17 infants, including 8 neonates. Presenting complaints that led to cardiology consultation were as follows: heart murmur in 16, near-syncope or syncope in 5, irregular cardiac rhythm in 4, palpitations in 4, fetal structural cardiac abnormalities in 5, fetal arrhythmias in 2, transient cyanosis in one, and chest pain in one. Demographics and echocardiographic parameters at the time of clinic presentation are listed in Table 1. Fourteen subjects (37%) did not have any other structural cardiac abnormality or dysrhythmia. Eight patients (21%) had secundum atrial septal defects with diameters from 3 to 11 mm with an average of 4.6 mm. An additional five patients exhibited a persistent left to right shunt through a patent foramen ovale beyond infancy with vena contracta diameters from 1 to 2 mm, and one of them also had an atrial septal aneurysm. Those with an atrial septal defect had significant dilation of the right atrium, tricuspid valve annulus, and right ventricle, along with increased indexed right ventricular free wall thickness and decreased left ventricular cavity diameter. Both groups with and without atrial septal defects had decreased mean pulmonary valve annulus diameter z-values and increased mean aortic valve annulus diameter z-values. Significant tricuspid valve regurgitation was present in six patients (16%); mild in four and moderate in two. Three patients (8%) had systolic doming of the pulmonary valve with trivial stenosis in two and severe stenosis in one patient, who also had atrial septal defects and moderate tricuspid valve regurgitation.
Table 1. Chiari network data at presentation
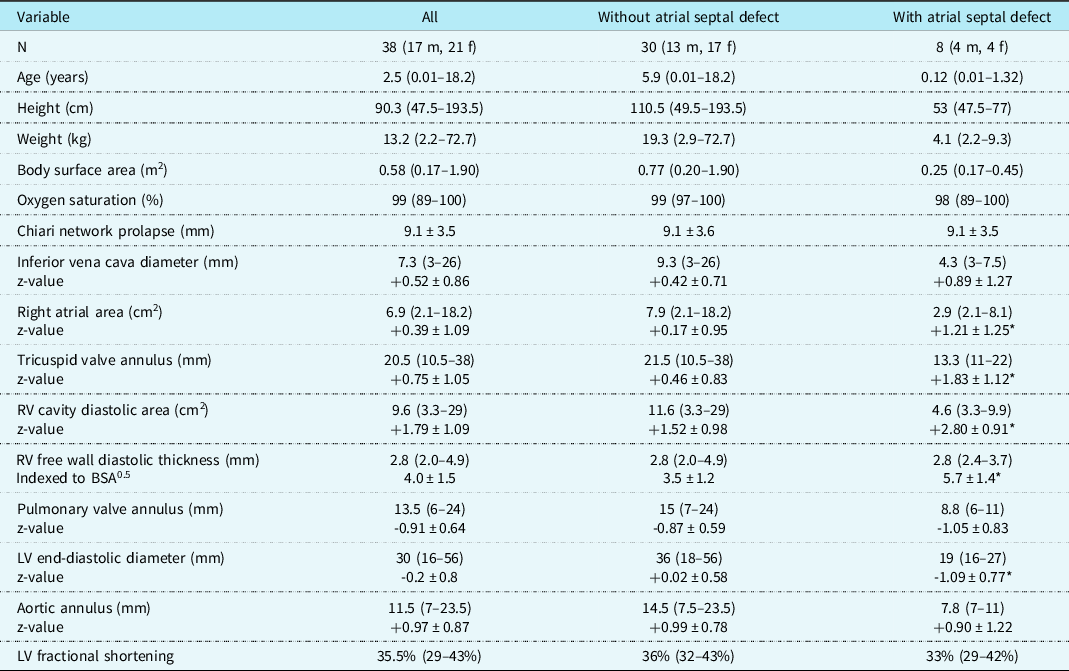
Values are median (range) or mean ± SD.
LV = left ventricle; RV = right ventricle.
*= p < 0.01 (with atrial septal defect versus without atrial septal defect).
Frequent premature atrial complexes were evident in eight patients (21%), mostly isolated and occasionally blocked or conducted with aberrancy. The two older patients presented with palpitations and the younger ones with an irregular heart rhythm. Three of these patients also had atrial septal defects and another had moderate tricuspid valve regurgitation. On Holter monitoring, the frequency of atrial ectopy ranged from 1.2 to 18.0%, median 1.8%. One patient had an atrial triplet. Supraventricular tachydysrhythmias were noted in three patients: one had sustained fetal atrial flutter just before birth at 36 weeks gestation, another had early neonatal sustained supraventricular tachycardia, and an adolescent developed recurrent sustained atrioventricular reentry tachycardia.
Follow-up evaluation
Fourteen of the original patients (37%) were reevaluated from 1 to 8.5 years (median 3.2 years) after the initial visit. Follow-up data were compared to that at presentation (Table 2). When followed serially, the length of Chiari network prolapse through the tricuspid annulus into the right ventricle decreased over time with growth. The low pulmonary valve diameter z-values increased, and the right ventricular end-diastolic area z-values decreased significantly toward mid-normal over this period. One patient had severe pulmonary valve stenosis requiring catheter balloon valvuloplasty at 1 day of age followed by later surgical excision of the prominent Chiari network, tricuspid annuloplasty for moderate regurgitation, and primary closure of three small secundum atrial septal defects at 3 years old. Two additional patients had doming pulmonic valves with initial trivial stenosis gradients that resolved over time. A total of six (43%) had secundum atrial septal defects at presentation with two spontaneously resolving during growth so far and two requiring closure, one by catheter device placement at 46 months old and the other by surgery as described above. Mild tricuspid valve regurgitation was evident at presentation in four patients (29%), which was unchanged at last evaluation in three with the other progressing to moderate and requiring surgical annuloplasty as outlined above. Additionally, one patient each had an atrial septal aneurysm that resolved by 25 months of age, a bicuspid aortic valve without stenosis or regurgitation, mild right upper pulmonary vein stenosis, mild aortic root dilation, and a small perimembranous ventricular septal defect that closed spontaneously by 22 months of age. Frequent atrial pre-mature complexes were present in two at presentation, which gradually disappeared spontaneously during growth. The infant with perinatal atrial flutter did not have a recurrence following urgent delivery. The infant with early neonatal sustained supraventricular tachycardia converted to sinus rhythm with vagal manoeuvres and was treated with propranolol therapy during the first year of life without reappearance thereafter. The adolescent with recurrent sustained atrioventricular reentry tachycardia underwent successful catheter radiofrequency ablation. Holter monitoring before and after ablation showed a persistent 1.2–1.5% burden of isolated pre-mature atrial complexes.
Table 2. Follow-up data compared to initial data
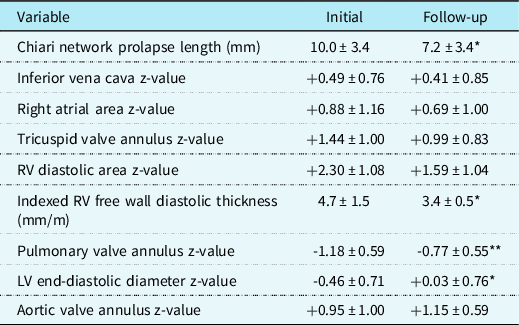
Values are mean ± SD.
LV = left ventricle; RV = right ventricle.
*p < 0.01 and **< 0.02 (Initial versus follow-up).
Using all patient data from presentation to last follow-up, there was a significant negative correlation between age and z-values for the right atrial area (r = -0.473), tricuspid valve annulus diameter (r = -0.520), and right ventricular diastolic area (r = -0.501) with p < 0.001 for all, as these values normalised over time (Fig 2). Intra-observer variability analysis of the echocardiographic measurements demonstrated Spearman and intra-class correlation coefficients > 0.95.
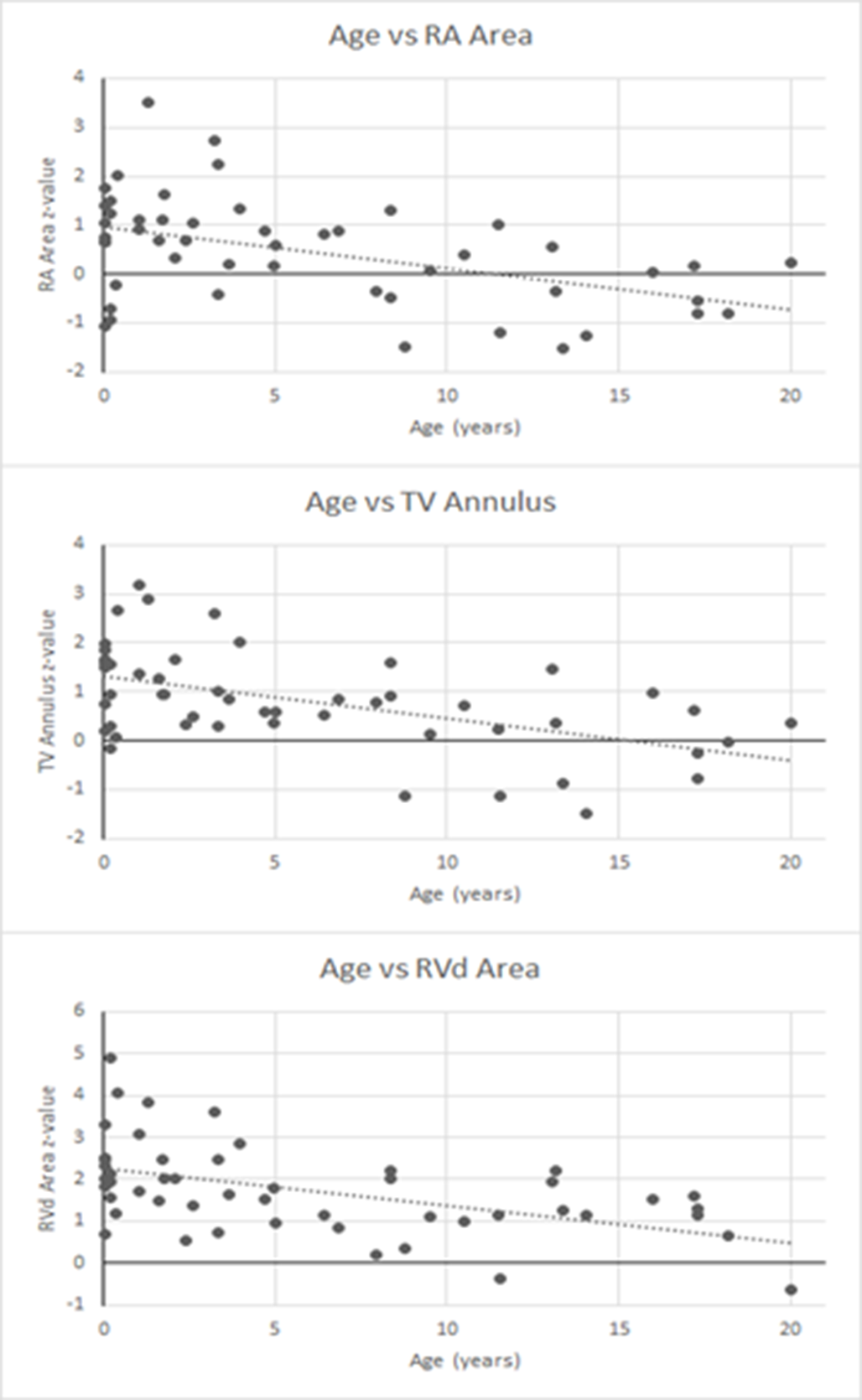
Figure 2. Graphs of age versus Z-values for right atrial (RA) area, tricuspid valve (TV) annulus diameter, and right ventricular diastolic (RVd) area using all data.
Discussion
Embryology and anatomy
The embryonic right valve of the sinus venosus protrudes into the right atrium towards the foramen ovale to direct the oxygen-rich blood from the inferior vena cava across the atrial septum to supply the left heart and upper body. Reference Moral, Ballesteros, Huguet, Panaro, Palet and Evangelista1-Reference Steding, Jinwen, Seidl, Männer and Xia2 This valve tissue regresses during later growth leaving remnants in the right atrium. Usually, these are seen as a haemodynamically insignificant Eustachian valve adjacent to the orifice of the inferior vena cava and a much smaller Thebesian valve next to the orifice of the coronary sinus on the atrial septum. Infrequently, with fragmentary resorption of the embryonic valve membrane, a network of interconnected filamentous strands can result which reaches from the inferior vena cava across the right atrium to the atrial septum and in some can elongate or stretch. Reference Bhatnagar, Nettleton, Campbell, Wagner, Kuwabara and Muresian3-Reference Klimek-Piotrowska, Hołda, Koziej and Strona5 Naito et al Reference Naito, Yu, Kim, Rodriguez-Vazquez, Murikami and Cho16 in a study of the topographical anatomy of the human fetal heart at around 8 weeks gestation detected variations in the anteroposterior arrangement of the coronary sinus, sinus septum, and right valve of the sinus venosus. They proposed that the right valve is more likely to be elongated postnatally, leading to the formation of a Chiari network, if the coronary sinus is adjacent to the right valve rather than the sinus septum at that early stage of development. On microscopic analysis in children and adults, the Chiari network has been found to consist of strands of cardiac muscle surrounded by elastic tissue. Reference Gresham17-Reference Suijker, Hazekamp, Rammeloo, Boon and Hruda18
Associated cardiac abnormalities
Cases have been reported of a prominent prolapsing Chiari network discovered as an incidental echocardiographic finding without other consequence. Reference Aypar, Sert and Odabaş9,Reference Blasco, Torres, Domenech, Blanch, Herrera and Comas19-Reference Pellett and Kerut20 However, there are also many case reports of this condition being associated with arrhythmias, Reference Suijker, Hazekamp, Rammeloo, Boon and Hruda18,Reference Clements, Sobotka-Plojhar, Exalto and Van Geijn21-Reference Nijres, Patel, Tay and Rodriguez23 atrial septal defects, Reference Chesi, Farinelli, Di, Marani and Reverzani7,Reference Suijker, Hazekamp, Rammeloo, Boon and Hruda18 tricuspid valve regurgitation Reference Suijker, Hazekamp, Rammeloo, Boon and Hruda18,Reference Chang24 , and significant obstruction to right ventricular outflow tract flow. Reference Heydarian, Siewers and Zuberbuhler25-Reference Moreno, Castro, Borches, Cerro, Garcia-Guereta and Rubio26 Additionally, Bendadi et al Reference Bendadi, van Tijn, Pistorius and Freund27 reported a case of early transient fetal hydrops from 12 to 20 weeks gestation that was due to a prolapsing Chiari network, along with another case of a term neonate with mild cyanosis (oxygen saturation 82–90%) that resolved by 4 months of age. They further noted, as we did, that the pulmonary valve annular diameters in both patients were initially small and later normalized with growth. Additional similar cases of transient neonatal cyanosis associated with a prolapsing Chiari network, some resolving within a few weeks, have been reported. Reference Aljemmali, Bokowski, Morales and Abdulla28 Large prolapsing Chiari networks were noted in a 10-week-old girl with frequent supraventricular tachycardia Reference Suijker, Hazekamp, Rammeloo, Boon and Hruda18 and an 11-year-old boy with recurrent paroxysmal atrial fibrillation, Reference Alegría-Barrero, Alegría-Barrero, Gavira Gómez and Rábago Juan-Aracil22 both of which resolved after surgical excision of the networks.
Our series, the largest collection of prominent prolapsing Chiari network in children, confirms the case report associations with atrial septal shunts, tricuspid valve regurgitation, supraventricular dysrhythmias, and pulmonic valve stenosis. It is reasonable to envision that a prominent Chiari network would direct more flow towards the fossa ovale and away from the right ventricular outflow tract. This increased flow across the atrial septum and away from the pulmonary valve could contribute to the development of an atrial septal defect and underdevelopment of the pulmonary valve. In support of this concept, we documented initially small pulmonary and large aortic valve annular diameters with gradual progression toward mid-normal during growth as the obstructing prolapsing Chiari network regressed. Additionally, an elongated Chiari network prolapsing onto and sometimes through the tricuspid valve could logically lead to the development of significant tricuspid valve regurgitation, which we noted in some patients. Lastly, with attachments from the orifice of the inferior vena cava across the atrial septum, the strands of cardiac muscle in the Chiari network have the potential to conduct electrical impulses and enhance the promotion of supraventricular arrhythmias. Of interest, a recent study of children in an outpatient clinic demonstrated an increase in the mean electrocardiographic P wave duration, amplitude, and dispersion associated with the presence of a Chiari network in the absence of any other cardiac abnormality. Reference Irdem, Akpinar, Celebi, Aygun and Dursun29 The gradual regression of the extent of Chiari network prolapse with growth during childhood and adolescence that we demonstrated may be due to an increase in the size of the atrium rather than a decrease in the length of the Chiari network. This slow regression likely explains why a prolapsing Chiari network is extremely rare in adults. However, less prominent Chiari networks can persist into adulthood and, if coincident with a patent foramen ovale, may be associated with an increased risk of developing a classic migraine with aura Reference Rigatelli, Dell’Avvocata and Cardiaoli30 and/or an embolic stroke. Reference Schneider, Hofmann, Justen and Meinertz8,Reference Nakayama, Takaya and Akagi31-Reference Rigatelli, Dell’Avvocata, Braggion, Giordan, Chinaglia and Cardaioli32 These tissue networks have also been reported to pose a challenge during cardiac catheterisation causing entanglement of atrial septal occluder devices Reference Cooke, Gelman and Harper33 and electrophysiology catheters. Reference Chu, Solheim, Chen, Hoff and Schuster34
Limitations
This study was retrospective, but the identification of a prominent Chiari network was logged in the echocardiogram reports at the time of the patient encounters prospectively, minimising any selection bias. The sample size of our study was small due to the rarity of the condition, and patient compliance with follow-up evaluations was incomplete. However, the age range spanned all of childhood and our analysis showed adequate power to detect statistically significant differences in some major variables.
Conclusions
The presence of an elongated prolapsing Chiari network is associated with an increased risk of having an atrial septal defect, supraventricular dysrhythmia, tricuspid valve regurgitation, and pulmonary valve stenosis. The extent of Chiari network prolapse across the tricuspid annulus gradually decreases during growth.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951121003668
Acknowledgements
We are grateful for the excellent work of our former and current lead cardiac sonographers: Amanda Whitlow, Shaina Jackson, and Ashley Sheehan.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None. The authors have no conflict of interest to disclose.









