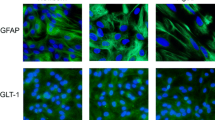
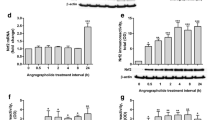
Abstract
Glucocorticoids (GCs) regulate astrocyte function, while glutamine synthetase (GS), an enzyme highly expressed in astrocytes, is one of the most remarkable GCs-induced genes. GCs mediate their effects through their cognate glucocorticoid receptor (GRα and GRβ isoforms); however, the mechanism via which these isoforms regulate GS activity in astrocytes remains unknown. We used dexamethasone (DEX), a classical GRα/GRβ agonist, RU486, which is a specific GRβ ligand, and Compound A, a known “dissociated” ligand, to delineate the mechanism via which GR modulates GS activity. Aged Mouse Cerebral Hemisphere astrocytes were treated with DEX (1 μM), RU486 (1 nM–1 μM) or compound A (10 μM), alone or in combination with DEX. GS activity and expression, GR isoforms (mRNA and protein levels), and GRα subcellular trafficking were measured. DEX increased GS activity in parallel with GRα nuclear translocation. RU486 increased GS activity in absence of GRα nuclear translocation implicating thus a role of GRβ-mediated mechanism compound A had no effect on GS activity implicating a GRα–GRE-mediated mechanism. None of the compounds affected whole-cell GRα protein content. DEX reduced GRα and GRβ mRNA levels, while RU486 increased GRβ gene expression. We provide evidence that GS activity, in astrocytes, is regulated via GRα- and GRβ-mediated pathways with important implications in pathological conditions in which astrocytes are involved.





Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- U-2Os:
-
Human bone osteosarcoma epithelial cells
- MEF cells:
-
Mouse embryonic fibroblast cells
- IM-9:
-
Human B-lymphoblastoid cells
- COS-1:
-
Fibroblast-like cell line from monkey kidney tissue
- HCT116:
-
Human colon cancer cell line
- FLS:
-
Fibroblast-like synoviocytes
- CEM-C7:
-
Human T-lymphoblast cell line
- BEAS-2B:
-
Human bronchial epithelial cell line
References
Rose CF, Verkhratsky A, Parpura V (2013) Astrocyte glutamine synthetase: pivotal in health and disease. Biochem Soc Trans 41:1518–1524. https://doi.org/10.1042/BST20130237
Cohen-Salmon M, Slaoui L, Mazaré N, Gilbert A, Oudart M, Alvear-Perez R, Elorza-Vidal X, Chever O, Boulay A-C (2020) Astrocytes in the regulation of cerebrovascular functions. Glia 69:1–25. https://doi.org/10.1002/glia.23924
Kitchen P et al (2020) Targeting aquaporin-4 subcellular localization to treat central nervous system edema. Cell 181:784-799.e19. https://doi.org/10.1016/j.cell.2020.03.037
Sylvain NJ, Salman MM, Pushie MJ, Hou H, Meher V, Herlo R, Peeling L, Kelly ME (2021) The effects of trifluoperazine on brain edema, aquaporin-4 expression, and metabolic markers during the acute phase of stroke using photothrombotic mouse model. (BBA)—Biomembranes 1863:183573. https://doi.org/10.1016/j.bbamem.2021.183573
Kimelberg HK (2010) Functions of mature astrocytes: a current view. Neuroscientist 16:79–106. https://doi.org/10.1177/1073858409342593
Verkhratsky A, Parpura V (2010) Recent advances in (patho)physiology of astroglia. Acta Pharmacol Sin 31:1044–1054. https://doi.org/10.1038/aps.2010.108
Ransohoff RM, Brown MA (2012) Innate immunity in the central nervous system. J Clin Invest 122:1164–1171. https://doi.org/10.1172/JCI58644
Hashioka S, Miyaoka T, Wake R, Furuya M, Horiguchi J (2013) Glia: an important target for anti-inflammatory and antidepressant activity. Curr Drug Target 14:1322–1328. https://doi.org/10.2174/13894501113146660214
Rodriguez-Arellano JJ, Parpura V, Zorec R, Verkhratsky A (2016) Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience 323:170–182. https://doi.org/10.1016/j.neuroscience.2015.01.007
Bellaver B, Souza DG, Souza DO, Quincozes-Santos A (2017) Hippocampal astrocyte cultures from adult and aged rats reproduce changes in glial functionality observed in the aging brain. Mol Neurobiol 54:2969–2985. https://doi.org/10.1007/s12035-016-9880-8
Barres BA (2008) The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60:430–440. https://doi.org/10.1016/j.neuron.2008.10.013
Verkhratsky A, Ho MS, Vardjan N, Zorec R, Parpura V (2019) General pathophysiology of astroglia. Adv Exp Med Biol 1175:149–179. https://doi.org/10.1007/978-981-13-9913-8_7
Araque A, Parpura V, Sanzgiri RP, Haydon PG (1999) Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22:208–221. https://doi.org/10.1016/s0166-2236(98)01349-6
Lewerenz J, Maher P (2015) Chronic glutamate toxicity in neurodegenerative diseases—what is the evidence? Front Neurosci 9:469. https://doi.org/10.3389/fnins.2015.00469
Bordone MP et al (2019) The energetic brain: a review from students to students. J Neurochem 151:139–165. https://doi.org/10.1111/jnc.14829
Hertz L, Bock E, Schousboe A (1978) GFA content, glutamate uptake and activity of glutamate metabolizing enzymes in differentiating mouse astrocytes in primary cultures. Dev Neurosci 1:226–238. https://doi.org/10.1159/000112577
Norenberg MD, Martinez-Hernandez A (1979) Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res 161(2):303–310. https://doi.org/10.1016/0006-8993(79)90071-4
Jayakumar AR, Norenberg MD (2016) Glutamine synthetase: role in neurological disorders. Adv Neurobiol 13:327–350. https://doi.org/10.1007/978-3-319-45096-4_13
Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, Myers RM, Bunney WE Jr, Akil H, Watson SJ, Jones EG (2005) Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci USA 102:15653–15658. https://doi.org/10.1073/pnas.0507901102
Rajkowska G, Stockmeier CA (2013) Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets 14:1225–1236. https://doi.org/10.2174/13894501113149990156
Virgin CE Jr, Ha TP, Packan DR, Tombaugh GC, Yang SH, Horner HC, Sapolsky RM (1991) Glucocorticoids inhibit glucose transport and glutamate uptake in hippocampal astrocytes: implications for glucocorticoid neurotoxicity. J Neurochem 57:1422–1428. https://doi.org/10.1111/j.1471-4159.1991.tb08309.x
Juurlink BHJ, Schousboe A, Jorgensen OS, Hertz L (1981) Induction by hydrocortisone of glutamine synthetase in mouse primary astrocyte cultures. J Neurochem 36:136–142. https://doi.org/10.1111/j.1471-4159.1981.tb02388.x
Patel AJ, Hunt A (1985) Observations on cell growth and regulation of glutamine synthetase by dexamethasone in primary cultures of forebrain and cerebellar astrocytes. Dev Brain Res 18:175–184. https://doi.org/10.1016/0165-3806(85)90262-7
Khelil M, Rolland B, Fages C, Tardy M (1990) Glutamine synthetase modulation in astrocyte cultures of different mouse brain areas. Glia 3:75–80. https://doi.org/10.1002/glia.440030110
Arcuri C, Tardy M, Rolland B, Armellini R, Menghini AR, Bocchini V (1995) Glutamine synthetase gene expression in a glioblastoma cell-line of clonal origin: regulation by dexamethasone and dibutyryl cyclic AMP. Neurochem Res 20:1133–1139. https://doi.org/10.1007/BF00995375
Huang TL, O’Banion MK (1998) Interleukin-1β and tumor necrosis factor-α suppress dexamethasone induction of glutamine synthetase in primary mouse astrocytes. J Neurochem 71:1436–1442. https://doi.org/10.1046/j.1471-4159.1998.71041436.x
Kumar S, Holmes S, Scully S, Birren BW, Wilson RH, de Vellis J (1986) The hormonal regulation of gene expression of glial markers: glutamine synthetase and glycerol phosphate dehydrogenase in primary cultures of rat brain and in C6 cell line. J Neurosci Res 16:251–264. https://doi.org/10.1002/jnr.490160122
O’Banion MK, Young DA, Bohn MC (1994) Corticosterone-responsive mRNAs in primary rat astrocytes. Mol Brain Res 22:57–68. https://doi.org/10.1016/0169-328X(94)90032-9
Patel AJ, Hunt A, Tahourdin CSM (1983) Regulation of in vivo glutamine synthetase activity by glucocorticoids in the developing rat brain. Dev Brain Res 312:83–91. https://doi.org/10.1016/0165-3806(83)90123-2
Zhang HY, Young AP (1991) A single upstream glucocorticoid response element juxtaposed to an AP1/ATF/CRE-like site renders the chicken glutamine synthetase gene hormonally inducible in transfected retina. J Biol Chem 266:24332–24338
Lu NZ, Cidlowski JA (2006) Glucocorticoid receptor isoforms generate transcription activity. Trends Cell Biol 16:301–307. https://doi.org/10.1016/j.tcb.2006.04.005
Kino T, Manoli I, Kelkar S, Wang Y, Su YA, Chrousos G (2009) Glucocorticoid receptor (GR) β has intrinsic, GRα-independent transcriptional activity. Biochem Biophys Res Commun 381:671–675. https://doi.org/10.1111/j.1471-4159.1981.tb02388.x
Nicolaides NC, Galata Z, Kino T, Chrousos GP, Charmandari E (2010) The human glucocorticoid receptor: molecular basis of biologic function. Steroids 75:1–12. https://doi.org/10.1016/j.steroids.2009.09.002
Nixon M, Andrew R, Chapman KE (2013) It takes two to tango: dimerisation of glucocorticoid receptor and its anti-inflammatory functions. Steroids 78:59–68. https://doi.org/10.1016/j.steroids.2012.09.013
Bamberger CM, Schulte HM, Chrousos GP (1996) Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocr Rev 17:245–261. https://doi.org/10.1210/edrv-17-3-245
Kassel O, Herrlich P (2007) Crosstalk between the glucocorticoid receptor and other transcription factors: molecular aspects. Mol Cell Endocrinol 275:13–29. https://doi.org/10.1016/j.mce.2007.07.003
Vegiopoulos A, Herzig S (2007) Glucocorticoids, metabolism and metabolic diseases. Mol Cell Endocrinol 275:43–61. https://doi.org/10.1016/j.mce.2007.05.015
Lewis-Tuffin LJ, Jewell CM, Bienstock RJ, Collins JB, Cidlowski JA (2007) Human glucocorticoid receptor β binds RU-486 and is transcriptionally active. Mol Cell Biol 27:2266–2282. https://doi.org/10.1128/MCB.01439-06
Oakley RH, Cidlowski JA (2011) Cellular processing of the glucocorticoid receptor gene and protein: new mechanisms for generating tissue-specific actions of glucocorticoids. J Biol Chem 286:3177–3318. https://doi.org/10.1074/jbc.R110.179325
Lewis-Tuffin LJ, Cidlowski JA (2006) The physiology of human glucocorticoid receptor beta (hGRbeta) and glucocorticoid resistance. Ann N Y Acad Sci 1069:1–9. https://doi.org/10.1196/annals.1351.001
Oakley RH, Sar M, Cidlowski JA (1996) The human glucocorticoid receptor beta isoform: expression, biochemical properties, and putative function. J Biol Chem 271:9550–9559. https://doi.org/10.1074/jbc.271.16.9550
Marques F, Sousa JC, Cerqueira JJ, Sousa N (2014) Detection of the glucocorticoid receptors in brain protein extracts by SDS-PAGE. Methods Mol Biol 1204:233–242. https://doi.org/10.1007/978-1-4939-1346-6_20
Yin Y, Zhang X, Li Z, Deng L, Jiao G, Zhang B, Xie P, Mu H, Qiao W, Zou J (2013) Glucocorticoid receptor β regulates injury-mediated astrocyte activation and contributes to glioma pathogenesis via modulation of b-catenin/TCF transcriptional activity. Neurobiol Dis 59:165–176. https://doi.org/10.1016/j.nbd.2013.07.013
Hinds TD Jr, Ramakrishnan S, Cash HA, Stechschulte LA, Heinrich G, Naijar SM, Sanchez ER (2010) Discovery of glucocorticoid receptor-β in mice with a role in metabolism. Mol Endocrinol 24:1715–1727. https://doi.org/10.1210/me.2009-0411
Fahrner J, Labruyere WT, Gaunitz C, Moorman AFM, Gebhardt R, Lamers WH (1993) Identification and functional characterization of regulatory elements of the glutamine synthetase gene from rat liver. Eur J Biochem 213:1067–1073. https://doi.org/10.1111/j.1432-1033.1993.tb17854.x
Chandrasekhar S, Souba WW, Abcouwer SF (1999) Identification of glucocorticoid-responsive elements that control transcription of rat glutamine synthetase. Am J Physiol 276:L319–L331. https://doi.org/10.1152/ajplung.1999.276.2.L319
Pu HF, Young AP (1990) Glucocorticoid-inducible expression of a glutamine synthetase-CAT-encoding fusion plasmid after transfection of intact chicken retinal explant cultures. Gene 89:259–263. https://doi.org/10.1016/0378-1119(90)90014-I
Vardimon L, Ben-Dror I, Avisar N, Oren A, Shiftan L (1999) Glucocorticoid control of glial gene expression. J Neurobiol 40:513–527. https://doi.org/10.1002/(sici)1097-4695(19990915)40:4%3c513::aid-neu8%3e3.0.co;2-d
Einstein M, Greenlee M, Rouen G, Sitlani A, Santoro J, Wang C, Pandit S, Mazur P, Smalera I, Weaver AP, Zeng YY, Ge L, Kelly T, Paiva T, Geissler W, Mosley RT, Williamson J, Ali A, Balkovec J, Harris G (2004) Selective glucocorticoid receptor nonsteroidal ligands completely antagonize the dexamethasone mediated induction of enzymes involved in gluconeogenesis and glutamine metabolism. J Steroid Biochem Mol Biol 92:345–356. https://doi.org/10.1016/j.jsbmb.2004.10.009
Sundahl N, Bridelance J, Libert C, De Bosscher K, Beck IM (2015) Selective glucocorticoid receptor modulation: new directions with non-steroidal scaffolds. Pharmacol Ther 152:28–41. https://doi.org/10.1016/j.pharmthera.2015.05.001
Unemura K, Kume T, Kondo M, Maeda Y, Izumi Y, Akaike A (2012) Glucocorticoids decrease astrocyte numbers by reducing glucocorticoid receptor expression in vitro and in vivo. J Pharmacol Sci 119:30–39. https://doi.org/10.1254/jphs.12047FP
Lou Y-X, Li J, Wang Z-Z, Xia C-Y, Chen N-H (2018) Glucocorticoid receptor activation induces decrease of hippocampal astrocyte number in rats. Psychopharmacology 235:2529–2540. https://doi.org/10.1007/s00213-018-4936-2
Allaman I, Pellerin L, Magistretti PJ (2004) Glucocorticoids modulate neurotransmitter-induced glycogen metabolism in cultured cortical astrocytes. J Neurochem 88:900–908. https://doi.org/10.1046/j.1471-4159.2003.02235.x
Zhang Y-P, Wang H-Y, Zhang C, Liu B-P, Peng Z-L, Li Y-Y, Liu F-M, Song C (2018) Mifepristone attenuates depression-like changes induced by chronic central administration of interleukin-1β in rats. Behav Brain Res 347:436–445. https://doi.org/10.1016/j.bbr.2018.03.033
Zschocke J, Bayatti N, Clement AM, Witan H, Figiel M, Engele J, Behl C (2005) Differential promotion of glutamate transporter expression and function by glucocorticoids in astrocytes from various brain regions. J Biol Chem 280:34924–34932. https://doi.org/10.1074/jbc.M502581200
De Bosscher K, Berghe WV, Beck IME, Van Molle W, Hennuyer N, Hapgood J, Libert C, Staels B, Louw A, Haegeman G (2005) A fully dissociated compound of plant origin for inflammatory gene repression. Proc Natl Acad Sci USA 102:15827–15832. https://doi.org/10.1073/pnas.0505554102
Dewint P, Gossye V, De Bosscher K, Berghe WV, Van Beneden K, Deforce D, Van Calenbergh S, Müller-Ladner U, Vander Cruyssen B, Verbruggen G, Haegeman G, Elewaut D (2008) A plant-derived ligand favoring monomeric glucocorticoid receptor conformation with impaired transactivation potential attenuates collagen-induced arthritis. J Immunol 180:2608–2615. https://doi.org/10.4049/jimmunol.180.4.2608
Wüst S, Tischner D, John M, Tuckermann JP, Menzfeld C, Hanisch U-K, van den Brandt J, Lühder F, Reichardt HM (2009) Therapeutic and adverse effects of a non-steroidal glucocorticoid receptor ligand in a mouse model of multiple sclerosis. PLoS ONE 4:e8282. https://doi.org/10.1371/journal.pone.0008202
Van Loo G, Sze M, Bougarne N, Praet J, Mc Guire C, Ullrich G, Haegeman G, Prinz M, Beyaert R, De Bosscher K (2010) Antiinflammatory properties of a plant-derived nonsteroidal, dissociated glucocorticoid receptor modulator in experimental autoimmune encephalomyelitis. Mol Endocrinol 24:310–322. https://doi.org/10.1210/me.2009-0236
Lesovaya E, Yemelyanov A, Swart AC, Swart P, Haegeman G, Budunova I (2015) Discovery of Compound A: a selective activator of the glucocorticoid receptor with anti-inflammatory and anti-cancer activity. Oncotarget 6:30730–30744. https://doi.org/10.18632/oncotarget.5078
Zhang Z, Zhang ZY, Schluesener HJ (2009) Compound A, a plant origin ligand of glucocorticoid receptors, increases regulatory T cells and M2 macrophages to attenuate experimental autoimmune neuritis with reduced side effects. J Immunol 183:3081–3091. https://doi.org/10.4049/jimmunol.0901088
Vernadakis A, Mangoura D, Sakellaridis N, Linderholm S (1984) Glial cells dissociated from newborn and aged mouse. J Neurosci Res 11:253–262. https://doi.org/10.1002/jnr.490110305
Vernadakis A, Fleischer-Lambropoulos H (2000) Cell culture as a model to study cell–cell interactions during development aging and neurodegenerative diseases. Int J Dev Neurosci 18:139–143. https://doi.org/10.1016/S0736-5748(99)00081-7
Vernadakis A, Davies D, Sakellaridis N, Mangoura D (1986) Growth patterns of glial cells dissociated from newborn and aged mouse brain with cell passage. J Neurosci Res 15:79–85. https://doi.org/10.1002/jnr.490150108
Lee K, Kentroti S, Billie H, Bruce C, Vernadakis A (1992) Comparative biochemical, morphological and immunocytochemical studies between C-6 glial cells of early and late passages and advanced passages of glial cells derived from aged mouse cerebral hemispheres. Glia 6:245–257. https://doi.org/10.1002/glia.440060402
Holbrook NJ, Grasso RJ, Hackney JF (1981) Glucocorticoid receptor properties and glucocorticoid regulation of glutamine synthetase activity in sensitive C6 and resistant C6H glial cells. J Neurosci Res 6(1):75–88. https://doi.org/10.1002/jnr.490060108
Berl S (1966) Glutamine synthetase. Determination of its distribution in brain development. Biochemistry 5:916–922. https://doi.org/10.1021/bi00867a016
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Xie J, Nair A, Hermiston TW (2008) A comparative study examining the cytotoxicity of inducible gene expression system ligands in different cell types. Toxicol In Vitro 22:261–266. https://doi.org/10.1016/j.jviromet.2008.07.005
Max SR (1990) Glucocorticoid-mediated induction of glutamine synthetase in skeletal muscle. Med Sci Sports Exerc 22:325–330
Sarantos P, Howard D, Souba WW (1993) Dexamethasone regulates glutamine synthetase expression in rat lung. Metabolism 42:795–800. https://doi.org/10.1016/0026-0495(93)90252-J
Feng B, Hilt DC, Max SR (1990) Transcriptional regulation of glutamine synthetase gene expression by dexamethasone in L6 muscle cells. J Biol Chem 265:18702–18706
Patel AJ, Hunt A, Faraji-Shadan F (1986) Effect of removal of glutamine and addition of dexamethasone on the activities of glutamine synthetase, ornithine decarboxylase and lactate dehydrogenase in primary cultures of forebrain and cerebellar astrocytes. Brain Res 391:229–238. https://doi.org/10.1016/0165-3806(86)90287-7
Max SR, Mill J, Mearow K, Konagaya M, Konagaya Y, Thomas JW, Banner C, Vitković L (1988) Dexamethasone regulates glutamine synthetase expression in rat skeletal muscles. Am J Physiol 255:E397–E402. https://doi.org/10.1152/ajpendo.1988.255.3.E397
Labow BI, Souba WW, Abcouwer SF (1999) Glutamine synthetase expression in muscle is regulated by transcriptional and posttranscriptional mechanisms. Am J Physiol 276:E1136–E1145. https://doi.org/10.1152/ajpendo.1999.276.6.E1136
Moutsatsou P, Kazazoglou T, Fleischer-Lambropoulos H, Psarra AMG, Tsiapara A, Sekeris CE, Stefanis C, Vernadakis A (2000) Expression of the glucocorticoid receptor in early and late passage C-6 glioma cells and in normal astrocytes derived from aged mouse cerebral hemispheres. Int J Dev Neurosci 18:329–335. https://doi.org/10.1016/S0736-5748(99)00102-1
Vielkind U, Walencewicz A, Levine JM, Bohn MC (1990) Type II glucocorticoid receptors are expressed in oligodendrocytes and astrocytes. J Neurosci Res 27:360–373. https://doi.org/10.1002/jnr.490270315
Dong Y, Poellinger L, Gustafsson J-A, Okret S (1988) Regulation of glucocorticoid receptor expression: evidence for transcriptional and posttranscriptional mechanisms. Mol Endocrinol 2:1256–1264. https://doi.org/10.1210/mend-2-12-1256
Hoeck W, Rusconi S, Groner B (1989) Down-regulation and phosphorylation of glucocorticoid receptors in cultured cells: investigations with a monospecific antiserum against a bacterially expressed receptor fragment. J Biol Chem 264:14396–14402
Freeman AI, Munn HL, Lyons V, Dammermann A, Seckl JR, Chapman KE, (2004) Glucocorticoid down-regulation of rat glucocorticoid receptor does not involve differential promoter regulation. J Endocrinol 183:365–374. https://doi.org/10.1677/joe.1.05773
Ramamoorthy S, Cidlowski JA (2013) Ligand-induced repression of the glucocorticoid receptor gene is mediated by an NCoR1 repression complex formed by long-range chromatin interactions with intragenic glucocorticoid response elements. Mol Cell Biol 33:1711–1722. https://doi.org/10.1128/MCB.01151-12
Ramdas J, Liu W, Harmon JM (1999) Glucocorticoid-induced cell death requires autoinduction of glucocorticoid receptor expression in human leukemic T cells. Cancer Res 59:1378–1385
Pedersen KB, Vedeckis WV (2003) Quantification and glucocorticoid regulation of glucocorticoid receptor transcripts in two human leukemic cell lines. Biochemistry 42:10978–10990. https://doi.org/10.1021/bi034651u
Pujols L, Mullol J, Pérez M, Roca-Ferrer J, Juan M, Xaubet A, Cidlowski JA, Picado C (2001) Expression of the human glucocorticoid receptor alpha and beta isoforms in human respiratory epithelial cells and their regulation by dexamethasone. Am J Respir Cell Mol Biol 24:49–57. https://doi.org/10.1165/ajrcmb.24.1.4024
Max SR, Thomas JW, Banner C, Vitkovic L, Konagaya M, Konagaya Y (1987) Glucocorticoid receptor-mediated induction of glutamine synthetase in skeletal muscle cells in vitro. Endocrinology 120:1179–1183. https://doi.org/10.1210/endo-120-3-1179
Carter BS, Meng F, Thompson RC (2012) Glucocorticoid treatment of astrocytes results in temporally dynamic transcriptome regulation and astrocyte-enriched mRNA changes in vitro. Physiol Genomics 44:1188–1200. https://doi.org/10.1152/physiolgenomics.00097.2012
Rajpert EJ, Lemaigre FP, Eliard PH, Place M, Lafontaine DA, Economidis IV, Belayew A, Martial JA, Rousseau GG (1987) Glucocorticoid receptors bound to the antagonist RU486 are not downregulated despite their capacity to interact in vitro with defined gene regions. J Steroid Biochem 26:513–520. https://doi.org/10.1016/0022-4731(87)90001-X
Alarid ET (2006) Lives and times of nuclear receptors. Mol Endocrinol 20:1972–1981. https://doi.org/10.1210/me.2005-0481
Robertson S, Allie-Reid F, Berghe WV, Visser K, Binder A, Africander D, Vismer M, De Bosscher K, Hapgood J, Haegeman G, Louw A (2010) Abrogation of glucocorticoid receptor dimerization correlates with dissociated glucocorticoid behavior of compound A. J Biol Chem 285:8061–8075. https://doi.org/10.1074/jbc.M109.087866
Wilkinson L, Verhoog N, Louw A (2018) Novel role for receptor dimerization in post-translational processing and turnover of the GRα. Sci Rep 8:14266. https://doi.org/10.1038/s41598-018-32440-z
Desmet SJ, Bougarne N, Van Moortel L, De Cauwer L, Thommis J, Vuylsteke M, Ratman D, Houtman R, Tavernier J, De Bosscher K (2017) Compound A influences gene regulation of the dexamethasone-activated glucocorticoid receptor by alternative cofactor recruitment. Sci Rep 7:8063. https://doi.org/10.1038/s41598-017-07941-y
Gossye V, Elewaut T, Van Beneden K, Dewint P, Haegeman G, De Bosscher K (2010) A plant-derived glucocorticoid receptor modulator attenuates inflammation without provoking ligand-induced resistance. Ann Rheum Dis 69:291–296. https://doi.org/10.1136/ard.2008.102871
Centeno EGZ, Cimarosti H, Bithell A (2018) 2D versus 3D human induced pluripotent stem cell-derived cultures for neurodegenerative disease modelling. Mol Neurodegener 13(1):27. https://doi.org/10.1186/s13024-018-0258-4
Ader M, Tanaka EM (2014) Modeling human development in 3D culture. Curr Opin Cell Biol 31:23–28. https://doi.org/10.1016/j.ceb.2014.06.013
DeBattista C, Belanoff J (2006) The use of mifepristone in the treatment of neuropsychiatric disorders. Trends Endocrinol Metab 17:117–121. https://doi.org/10.1016/j.tem.2006.02.006
Block T, Petrides G, Kushner H, Kalin N, Nelson C, Belanoff J, Schatzberg A (2018) Combined analysis of mifepristone for psychotic depression: plasma levels associated with clinical response. Biol Psychiatry 84:46–54. https://doi.org/10.1097/JCP.0000000000000744
Funding
This research was supported by the University Mental Health, Neurosciences and Precision Medicine Research Institute “Costas Stefanis” (UMHRI) and the Medical School, National and Kapodistrian University of Athens, University General Hospital “ATTIKON.”
Author information
Authors and Affiliations
Contributions
PM and TK contributed to the study concept and design. Material preparation, data collection, and analysis were performed by TK. Experiments were performed by TK, CP, CM, IK, and AP. Statistical analysis was carried out by CP. The first draft was written by TK. Writing and Review was performed by PM. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kazazoglou, T., Panagiotou, C., Mihailidou, C. et al. Glutamine synthetase regulation by dexamethasone, RU486, and compound A in astrocytes derived from aged mouse cerebral hemispheres is mediated via glucocorticoid receptor. Mol Cell Biochem 476, 4471–4485 (2021). https://doi.org/10.1007/s11010-021-04236-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-021-04236-9