Abstract
Cobalt oxide (Co3O4) thin films were fabricated by the drop-dry deposition (DDD) method using an aqueous solution containing Co(NO3)2 and NaOH. DDD was performed by dropping the solution on a substrate, heating-drying, and rinsing in water. The as-deposited film was Co(OH)2, and it was converted to Co3O4 by annealing in air. The Raman and x-ray diffraction peaks of Co3O4 were observed for the annealed films. We also fabricated Co3O4/ZnO heterojunction solar cells with ZnO prepared by the electrochemical deposition method. The heterojunction had rectification properties and a weak photovoltaic response. Thus, DDD is promising for fabricating Co3O4 layers, and the Co3O4/ZnO heterojunction can be used as a solar cell.
Export citation and abstract BibTeX RIS
1. Introduction
The depletion of traditional energy sources and the increasing environmental pollution problems have posed severe challenges to human economic activities and social life. It is particularly urgent to develop renewable energy sources to replace non-renewable energy sources. At present, crystalline silicon solar cells have been commercialized, with mature production processes and high conversion efficiency. However, due to the high purity requirements of silicon cells, the process is cumbersome, and the production cost remains high. In addition, the photoelectric conversion efficiency of silicon solar cells has an upper limit, and there is little room for improvement. Therefore, researchers continue to explore new processes and new materials, hoping to develop cheap and efficient solar cells. In recent years, metal oxide thin films have attracted attention of researchers. Many metal oxide materials are pollution-free and rich in reserves, meeting the requirements of low-cost manufacturing methods. There are a number of research works attempting to apply metal oxide materials for photovoltaics, such as TiO2 [1], ZnO [2] and Cux O [3–5], etc.
This study focuses on cobalt oxide (Co3O4). Co3O4 is a p-type metal oxide semiconductor and has two direct optical bandgaps of 1.5 eV and 2.0 eV [6–9]. Co3O4 has the spinel structure, in which there are two inequivalent Co atom sites (Co3+ and Co2+). The lower bandgap is considered to correspond to O2−–Co3+ charge transfer, and the higher one O2−–Co2+ charge transfer [6, 8, 9]. Co3O4 has already been used for a photo-electro-catalyst [10–12], CO gas sensor [13, 14], cathode material [15, 16] and photovoltaic device [17–20]. The bandgap of 1.5 eV is close to the optimum value for the solar cell application, and the theoretical upper-limit efficiency of Co3O4 cells is slightly higher than that of Si cells [21]. The detailed balance limit of efficiency is about 29.5% for Si and 30% for a material with a band gap of 1.5 eV [21]. In addition, cobalt is abundant and inexpensive: its elemental abundance in the earth crust is comparable to zinc and copper. Therefore, Co3O4 is well suited for an absorber material in solar cells. Since Co3O4 is inherently p-type, for photovoltaic application, a p-n heterojunction was formed with an n-type material, such as TiO2 and ZnO [17–20].
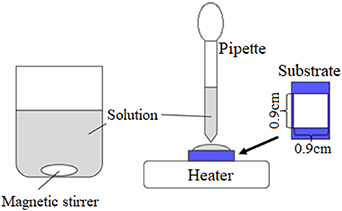
Co3O4 thin films have been fabricated by various methods, including chemical vapor deposition [6, 22], spray pyrolysis [23, 24], electrochemical deposition (ECD) [7, 25–28], sol-gel technique [29, 30], etc. In this work, we prepare Co(OH)2 thin films by drop-dry deposition (DDD) method, and transform it into Co3O4 films by an annealing treatment. DDD is a method of depositing a thin film by dropping and drying a solution on the substrate as shown in figure 1. It uses a heating plate only and does not need other apparatus, e.g. vacuum chamber, electric power supply, or light source. Thus the apparatus required by DDD is simple, easy to use, and therefore DDD is advantageous for deposition of a large area thin film at low cost. So far, DDD has been used for Mg(OH)2 only [31], but it is also potentially applicable for other hydroxides. This paper is the first report of application of DDD for a hydroxide other than Mg(OH)2. In this work, we also fabricate Co3O4/ZnO heterojunction solar cells with ZnO prepared by ECD. As shown below, photovoltaic output is confirmed, which demonstrates that DDD can be actually used for solar cell fabrication.
Figure 1. Apparatus of the drop-dry deposition method.
Download figure:
Standard image High-resolution image2. Experimental
For the Co(OH)2 preparation, 20 mM Co(NO3)2 and 10 mM NaOH were dissolved in pure water. (Under the premise that no obvious precipitates were produced in the solution, we also performed deposition with lower NaOH concentrations, and found that deposition results are rather insensitive to NaOH concentration.) After mixing, Co(OH)2 was spontaneously synthesized by the reaction
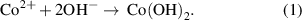
The substrate was an indium-tin-oxide (ITO)-coated glass sheet. Before the thin film deposition, the substrate was degreased and washed with acetone and pure water, and the deposition area was limited to 0.9 × 0.9 cm2 by masking. The deposition solution was dropped on the substrate using a pipette. Then the substrate was heated at 60 °C using a heater plate until it was visually observed that water was evaporated completely. Then, the substrate was rinsed with pure water and blown by a nitrogen gas. During the drying process, Co(OH)2, which has low solubility in water, first precipitates and is deposited on the substrate. After that, other solutes, having higher solubility, precipitates on the film, and then they are washed away in the subsequent rinsing process. The steps of the solution dropping and drying were repeated 5 times to deposit the Co(OH)2 thin film. Thermal decomposition of the Co(OH)2 films to Co3O4 was done by annealing the films at 200 °C or 400 °C for 1 h in air.
The Co3O4/ZnO heterojunction was fabricated by the following process. First, n-type ZnO was deposited on the ITO substrate by ECD, and then Co(OH)2 was deposited on it. The deposited area of ZnO was 1 × 1 cm2, and Co3O4 was 0.9 × 0.9 cm2. The deposition solution of ZnO contained 100 mM Zn(NO3)2 · 6H2O, and the current density was −1.5 mA cm−2; the chemical reactions of the deposition were shown in [32]. The deposition time was 10 min, and the temperature 60 °C. After the deposition of Co(OH)2, the heterojunction was annealed in air at 400 °C for 1 h.
Accretech Surfcom-1400D profiler was used to measure film thickness. Jasco V-570 UV/VIS/NIR spectrometer was used to measure the optical transmittance of the films. Auger electron spectroscopy (AES) data and scanning electron microscope (SEM) images were obtained using a JEOL JAMP-9500F field emission microprobe at a probe voltage of 10 keV. Jasco NRS-3300 Raman spectrometer was used to measure the Raman spectrum with excitation laser wavelength of 532 nm. X-ray diffraction (XRD) experiment was performed with a SmartLab SE x-ray diffractometer (Rigaku) using a Cu Kα source. Photoelectrochemical (PEC) measurements were performed with an Ag/AgCl reference electrode and 100 mM Na2SO3 solution electrolyte to determine the conductivity type. For optical excitation, AM1.5 light (100 mW cm−2) from the ABET Technologies 10 500 Solar Simulator was intermittently irradiated at 5 s intervals. The sample potential was varied at a scanning rate of 5 mV s−1 within a range from −1 to 1 V. For the current density-voltage (J–V) characterization, indium electrodes were fabricated by vacuum evaporation on the heterojunction, and then the J–V measurement was performed in the dark and under AM1.5 light irradiated on the substrate (ZnO) side of the sample.
3. Results and discussion
The thin films thickness was measured before and after annealing. The film thickness was about 0.5 μm before annealing. After annealing, the film thickness decreased to about half, about 0.25 μm. This is because Co(OH)2 was converted to Co3O4 after annealing. The theoretical volume change can be calculated using the molar mass and densities of Co(OH)2 and Co3O4. From the calculation result, the volume of the film after annealing was expected to be 0.52 times of that before annealing, which was consistent with the experimental results.
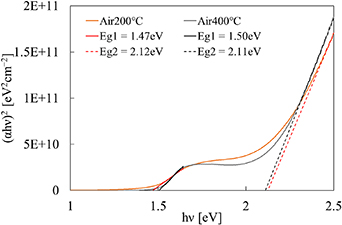
Figure 2 shows the optical transmittance of the thin films before and after annealing. It can be seen that in the film after annealing, there were absorption edges around 580 and 800 nm, which were absent for the film before annealing. It was due to the thermal transformation from Co(OH)2 to Co3O4. The bandgap calculation results are shown in figure 3. The band structure of Co3O4 is considered to be direct [6, 8], and thus the band gap can be evaluated by plotting (αhν)2 vs hν, where α is the absorption coefficient and hν is the photon energy. The bandgaps for the annealed films were found to be around 1.5 and 2.1 eV, which closely match the reported values [6]. As noted in the introduction, the first bandgap is considered to be due to O2−–Co3+ transition and the second one O2−–Co2+ transition [6, 8, 9].
Figure 2. Optical transmittance measurement results of the film before annealing (Co(OH)2) and those after annealing (Co3O4).
Download figure:
Standard image High-resolution imageFigure 3. Bandgap evaluation of the Co3O4 films after annealing.
Download figure:
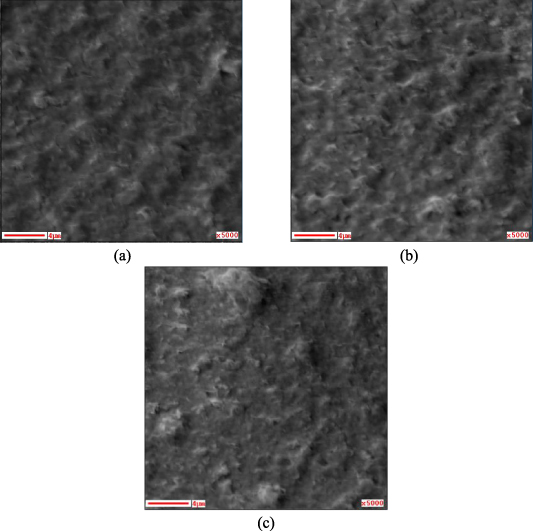
Standard image High-resolution imageFigure 4 shows the SEM images of the thin films before and after annealing. As the temperature of the annealing increases, the particulate structure gradually became visible, which would be due to the transformation of Co(OH)2 into Co3O4.
Figure 4. SEM images of the films before and after annealing: (a) the as-deposited Co(OH)2 film, (b) the Co3O4 film annealed at 200 °C, (c) annealed at 400 °C.
Download figure:
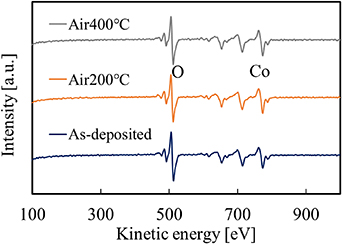
Standard image High-resolution imageFigure 5 shows the results of the AES measurement before and after annealing. Ar ion sputtering was performed for 10 s before the measurement. Co and O peaks were observed before and after annealing. The composition ratio was estimated using commercially available Co3O4 chemical as the reference. The composition ratio O/Co of the as-deposited film was 1.51, and those of the films after annealing at 200 °C and 400 °C were about 1.23 and 1.21, respectively. The composition ratio O/Co before annealing was considerably smaller than the stoichiometric composition ratio 2. This would be due to partial decomposition of hydroxide by the Ar sputtering.
Figure 5. AES spectra of the films before and after annealing.
Download figure:
Standard image High-resolution imageThe results of the Raman measurement before and after annealing are shown in figure 6. The peak of Co3O4 was confirmed from all the annealed thin films [33]. Thus, it was found that the thin film was converted to Co3O4 by annealing. However, we did not find the peak of Co(OH)2 before annealing [34].
Figure 6. Raman spectra of the films before and after annealing.
Download figure:
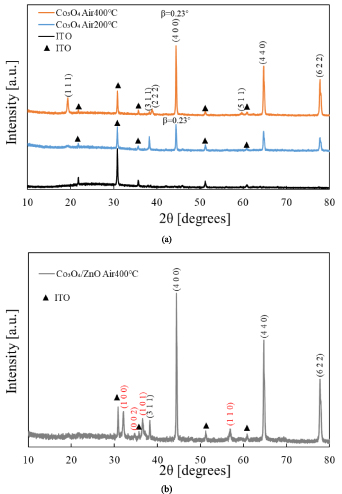
Standard image High-resolution imageXRD measurement was performed on the film annealed at 400 °C, and the results are shown in figure 7. With reference to the powder diffraction file of ICDD 00-009-0418, the main peaks observed correspond to the (222), (400), (440) and (622) peaks of Co3O4 in the spinel structure. The peaks of ITO are also observed: the XRD spectrum for the ITO substrate is also shown for comparison.
Figure 7. The XRD results of (a) the ITO substrate and the Co3O4 films after annealing, (b) the Co3O4/ZnO heterojunction after annealing. (The black indices are for Co3O4 and the red ones for ZnO).
Download figure:
Standard image High-resolution imageThe crystallite size D of the films was estimated using the Scherrer equation [35, 36]:
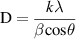
where k is the dimensionless shape factor (if β is the full width at half maximum, k = 0.89), λ the x-ray wavelength (0.154 nm), β the full width at half maximum, and θ the peak position. It can be seen from the figure 7(a) that the (400) peak was clearly observed for both the films annealed at 200 and 400 °C, and thus D was estimated using the (400) diffraction. According to the Scherrer equation, the crystallite size was about 37 nm for both. Thus, no significant difference was found by XRD for the films annealed at different temperatures.
The PEC measurement was performed on the sample after annealing. The results are shown in figure 8. If the sample is a p-type (n-type) semiconductor, significant photo response appears under negative (positive) potential. As shown in the figure, the p-type photo response was observed for the thin films annealed. Thus, the spinel p-type Co3O4 thin films were successfully fabricated by DDD.
Figure 8. PEC measurement results of the Co3O4 films after annealing.
Download figure:
Standard image High-resolution imageSince the p-type Co3O4 films was successfully prepared, we fabricated a heterojunction with n-type ZnO and tested its photovoltaic characteristics. The thickness of the ZnO prepared by ECD was about 1 μm, and the thickness of Co3O4 was about 0.25 μm after annealing. The XRD results for the annealed heterostructure are shown in figure 7(b). In addition to the Co3O4 and ITO peaks, the peaks due to wurtzite ZnO are observed. The (100) peak is most intense, which indicates weak preferential orientation of the ( ) plane.
) plane.
Figure 9 shows the J–V characteristics of the Co3O4/ZnO heterojunction in the dark and under AM1.5. Rectification properties and photo-responsivity were observed. In addition, it has a weak photovoltaic response, and its open-circuit voltage Voc was about 14 mV and the short-circuit current Jsc about 20 μA cm−2. This proves that the Co3O4/ZnO heterojunction can be used as a solar cell. The fill factor (FF) was estimated to be 0.25, and the energy-conversion efficiency was about 7.0 × 10−5%. We analyzed J–V on the basis of the conventional diode model, and found that shunt resistance (leakage current) seriously affected J–V, reducing Voc and FF.
Figure 9. J–V measurement results of the Co3O4/ZnO heterojunction annealed at 400 °C in the dark and under AM 1.5: (a) −1∼1 V, (b) −5∼20 mV.
Download figure:
Standard image High-resolution imageAlthough other researchers have prepared the Co3O4/ZnO heterojunction and measured its current–voltage characteristics, the reported rectification properties are not good [37, 38]. To improve diode properties, a MoO3 overlayer was deposited on Co3O4 [37], or an Al2O3 interface layer was inserted [38]. In contrast to their reports, the heterostructure fabricated in this work showed fairly good rectification properties without any over layer or interfacial layer. This may indicate that one can deposit Co(OH)2 layer by DDD without severely damaging the underlying ZnO layer so that less defective interface was formed. However, as noted above, the diode leakage current is still so large that the solar cell parameters are seriously affected. For future study, the interfacial defect formation need to be further suppressed for improving solar cell performance. In addition, for more detailed analyses of carrier transport across the interface, the band offsets between Co3O4 and ZnO need to be investigated since no experimental and theoretical works have been done for the band alignment of Co3O4/ZnO.
Although majority of the existing researches have used Co3O4 as a catalyst, the present results show that the Co3O4/ZnO heterojunction can be used as a solar cell. Moreover, it has been demonstrated for the first time that DDD can be successfully used for heterostructure fabrication. DDD is simple and low-cost, and thus will be advantageous for preparing a film for solar cells.
4. Conclusion
In this study, Co3O4 thin films were prepared by DDD and annealing. The deposition solution of DDD was an aqueous solution containing Co(NO3)2 and NaOH. The Raman and XRD peaks of Co3O4 were observed for the films after annealing. And the PEC measurement results showed that the Co3O4 film was a p-type semiconductor. The J–V measurement results showed that the Co3O4/ZnO heterojunction had rectification and photovoltaic properties. Thus, the Co3O4/ZnO heterojunction can be used as a solar cell, and DDD is potentially advantageous for solar cell fabrication.
Acknowledgments
Technical assistances of Ms Yuzuki Tomita and Mr Nor Mohd Ikram Bin Saad in the early stage of this work are acknowledged.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).