Abstract
Changes in photoluminescence (PL) peak width and quantum yield (PLQY) with halide composition were compared for CsPb(Br1−xIx)3 nanocrystals (NCs) prepared by hot-injection and ion-exchange methods. The hot-injection method produced single-phase cubic CsPb(Br1−xIx)3 NCs for each iodide ratio. In contrast, a small amount of orthorhombic phase was observed at higher x for NCs prepared by the ion-exchange methods. The PL peak width of mixed-halide NCs at x = 0.55 was broader than those of CsPbBr3 and CsPbI3, possibly as a result of compositional inhomogeneity among the individual NCs. However, the maximum PL peak width occurred at larger x for the ion-exchange methods, possibly because of partial transition to orthorhombic phase from cubic phase for CsPb(Br1−xIx)3 NCs. The minimum PLQY for the hot-injection method was 25.0% at x = 0.50, which was lower than the PLQYs of CsPbBr3 and CsPbI3 NCs (∼70%). In contrast, the minimum PLQY for the ion-exchange methods occurred at x = ∼0.3. Furthermore, the minimum PLQY was ∼10%, which was lower than 25.0% for the hot-injection method. The addition of halide solution for ion exchange might affect the adsorption state of surface modifiers, leading to the decreased PLQY.
Export citation and abstract BibTeX RIS
Next-generation displays require new luminescent materials to achieve the wide color gamut of Rec. 2020. Investigations on narrow emission peak width should be an important part of progress in display development. CsPbBr3 perovskite nanocrystal (NC) is a fascinating nanophosphor because of its excellent photoluminescence (PL) properties, 1 including green emission with a sharp peak, a high PL quantum yield (PLQY), and good durability when compared with organic–inorganic perovskite phosphors. As a result, many researchers have been attracted to investigations of this material, 2–5 and recent work has contributed to its development. 6–9 The ability to tune the emission wavelength of the material by changing the bandgap through halide composition is expected to open a wide range of applications. 8,10–13 Substitution of iodide ion for bromide ion in CsPbBr3 NC decreases the bandgap, leading to redshifts of the absorption edge and PL wavelength. CsPb(Br,I)3 NCs provide green, yellow, and red emissions with high color purities because of their narrow emission peaks. One of the most attractive applications of CsPbBr3 NC is a wide color gamut display with green-emitting phosphors. To achieve the chromaticity coordinates of the green vertex of the Rec. 2020 standard, adjustment of PL color by partial replacement of bromide ion with iodide ion in CsPbBr3 is needed. 8
In our previous study, CsPb(Br1−x Ix )3 NCs were prepared using a hot-injection method. 8 Their bandgaps and emission colors were changed with changing halide composition as reported by many researchers. In the present study, we observed interesting behavior of the full width at half-maximum (FWHM) of the PL peak for prepared NCs. Mono-halide NCs CsPbBr3 and CsPbI3 had narrow PL peak widths; however, the FWHM increased by mixing the halide ions in CsPb(Br1−x Ix )3 NCs. The broadest PL peak was observed at the molar ratio of Br:I = 0.5:0.5. Furthermore, PLQY was also affected by the changed halide composition. The lowest PLQY was achieved at Br:I = 0.5:0.5. Another major process to prepare mixed-halide NCs is an ion-exchange method using pre-synthesized mono-halide NCs. 14 Both preparation methods would result in different halide ion distributions and defects in obtained NCs which affect PL properties. In this work, the authors compare changes in FWHM and PLQY with halide composition for CsPb(Br1−x Ix )3 NCs prepared by hot-injection and ion-exchange methods.
Experimental
Materials
Cs2CO3 (99.99%, Mitsuwa Pure Chemical), PbBr2 (99%, Mitsuwa Pure Chemical), and PbI2 (99%, Sigma-Aldrich) powders were used as received without further purification. In addition, 1-octadecene (ODE; >90.0%, Tokyo Chemical Industry), oleic acid (OA; >85.0%, Tokyo Chemical Industry), oleylamine (OAm; 80%–90%, Acros Organics), tert-butyl alcohol (>99.0%, Tokyo Chemical Industry), methyl acetate (99.5%, Kanto Chemical), and toluene (99.5%, Kanto Chemical) were dehydrated over molecular sieves (3 A 1/8, Wako Pure Chemical Industries) prior to use.
Preparation of Cs-oleate precursor
Cs2CO3 (1.25 mmol), ODE (20.0 ml), and OA (1.3 ml) were mixed and degassed for 1 h at 120 °C and then purged with Ar gas. A Cs-oleate precursor solution was obtained by heating the mixture to 150 °C. The resulting solution was preheated at 100 °C before being injected into the reaction flask.
Synthesis of CsPb(Br1−xIx)3 NCs by hot-injection method
The hot-injection method by Kovalenko's group is the most famous synthetic method for CsPb(Br1−x Ix )3 NCs and has been well reported in the literature. 15,16 ODE (5.0 ml), 0.376 (1–x) mmol PbBr2, and 0.376 x mmol PbI2 (x = 0, 0.30, 0.33, 0.40, 0.45, 0.50, 0.55, 0.60, 0.80, and 1.00) were loaded into a four-neck flask and degassed for 1 h at 120 °C and then purged with Ar gas. OA (1.0 ml) and OAm (1.0 ml) were injected into the flask, and after completely dissolving the salt mixture, the temperature was raised to 180 °C. The Cs-oleate precursor solution (0.8 ml) was swiftly injected into the reaction flask and then cooled in an ice water bath after 5 s. Crystallized nanoparticles were precipitated by adding tert-butyl alcohol (25.0 ml). Alternatively, methyl acetate (25.0 ml) was used when x = 1.00. The NCs were collected by centrifugation at ∼11,000 g (10,000 rpm using a 10-cm-diameter rotor) for 5 min and then redispersed in toluene. The purified NC dispersion was used for optical analyses. Subsequently, the NC powder used for X-ray analyses was prepared by drying the collected NCs under vacuum overnight.
Preparation of CsPb(Br1−xIx)3 NCs from monohalide NCs CsPbBr3 and CsPbI3 by halide-exchange method
Crude dispersions of CsPbBr3 NC and CsPbI3 NC prepared by the hot-injection method were used to prepare mixed-halide NCs through halide exchange. PbBr2 or PbI2 (1.00 mmol) was dissolved in a mixture of OA (2.0 ml), OAm (2.0 ml), and toluene (6.0 ml) under stirring for 1 h at 80 °C. The desired volume of the obtained solution at room temperature was used to adjust the halide ratio of NCs, as summarized in Table I. After cooling in an ice water bath, the halide solution was added to the crude dispersion (2.0 ml) with vigorous stirring at room temperature. Herein, PbBr2 and PbI2 solutions were added to the crude dispersions of CsPbI3 NC and CsPbBr3 NC, respectively. After 10 min, ion-exchanged NCs were precipitated by adding tert-butyl alcohol (25.0 ml). Purified toluene dispersions and NC powders were prepared for analyses in the same way as described above.
Table I. Volumes of halide solutions used in the ion-exchange methods.
| Iodide ratio x in CsPb(Br1−x Ix )3 | Added PbI2 solution (ml) to CsPbBr3 NC dispersion | Added PbBr2 solution (ml) to CsPbI3 NC dispersion |
|---|---|---|
| 0.30 | 0.41 | 2.25 |
| 0.40 | 0.64 | 1.34 |
| 0.50 | 0.96 | 0.96 |
| 0.60 | 1.45 | 0.64 |
| 0.70 | 2.25 | 0.41 |
| 0.80 | 3.86 | 0.24 |
Characterization
The elemental ratios of the NC powders were measured using an X-ray fluorescence (XRF) analyzer (ZSXmini II, Rigaku). Elemental compositions were determined by the fundamental parameter method. Powder X-ray diffraction (XRD) profiles were obtained using an X-ray diffractometer (Rint-2200, Rigaku) with a Cu Kα radiation source and monochromator. The NC morphologies were observed by field-emission transmission electron microscopy (TEM; Tecnai G2, FEI). The TEM samples were prepared by vacuum drying a drop of NC dispersion on carbon-reinforced collodion-coated copper grids (COL-C10, Oken Shoji) overnight. UV–vis absorption spectra of the NC dispersions were measured using an optical absorption spectrometer (V-570, JASCO). The bandgaps of NCs were estimated from Tauc plots derived from the UV–vis spectra. 17 The PL spectra of the NC dispersions were measured using a fluorescence spectrometer (FP-6500, JASCO). Each spectral response was calibrated using an ethylene glycol solution of rhodamine B (5.5 g l−1) and a standard light source (ESC-333, JASCO). PL decay curves were recorded on a fluorescence lifetime spectrometer (Quantaurus-Tau C11367, Hamamatsu Photonics) using 405 nm LED. Herein, the PL decay curves were fitted with the following biexponential equation:
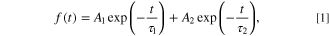
where A1 and A2 are the amplitudes, and τ1 and τ2 are the PL decay times. The average PL decay time 〈τ〉 was calculated using the following equation.
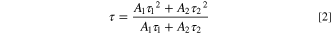
Absolute PLQYs were measured using a quantum efficiency measurement system (QE-2000–311C, Otsuka Electronics).
Results and Discussion
Structure and particle morphology
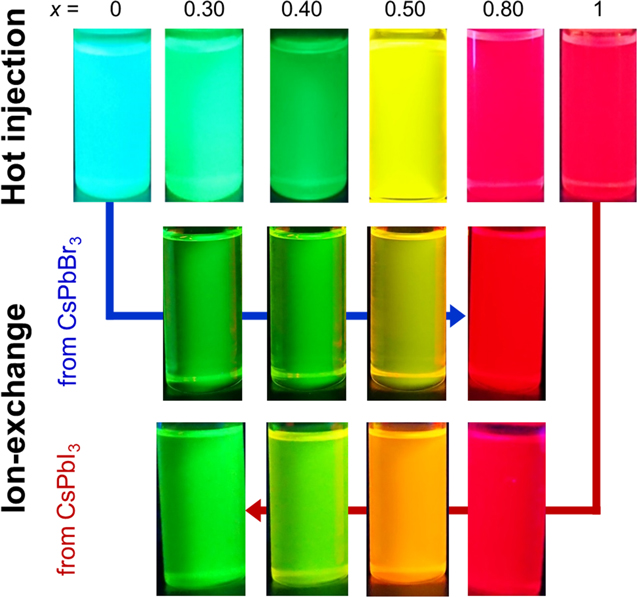
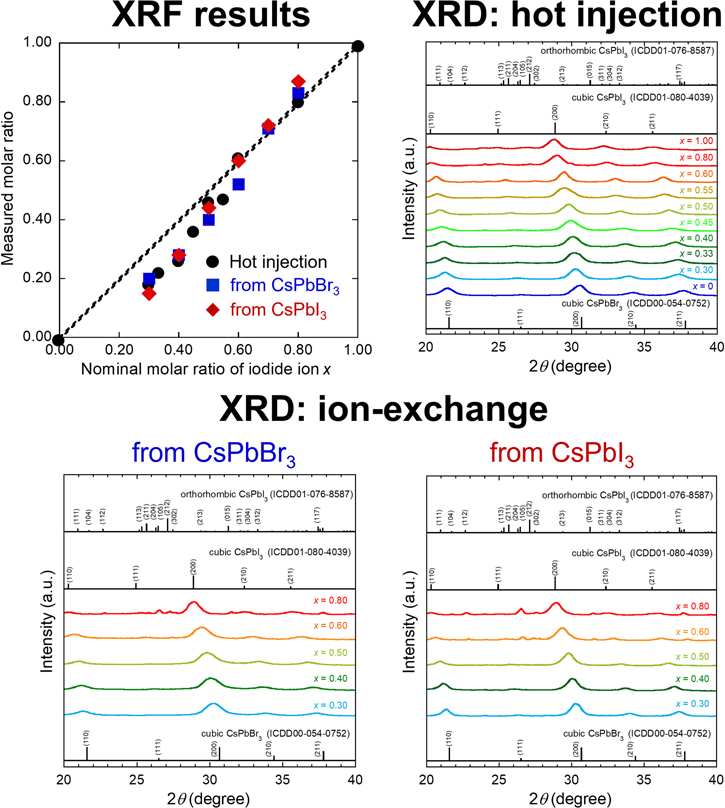
The emission color of prepared CsPb(Br1−x Ix )3 NCs changed from green to red with increasing x, as shown in Fig. 1. Their emission colors revealed that the mixed halides were successfully obtained. XRF results in Fig. 2 show a monotonic increase in actual iodide ratio with the nominal ratio. It should be noted that the measured ratios were lower than the nominal ratios in the Br-rich region regardless of preparation methods. This may be a result of errors derived from matrix effects in the fundamental parameter method of XRF.
Figure 1. Photographs of prepared CsPb(Br1−x Ix )3 NC dispersions in toluene under 365-nm UV light.
Download figure:
Standard image High-resolution imageFigure 2. XRF results and XRD profiles of CsPb(Br1−x Ix )3 NCs prepared by hot-injection method and ion-exchange methods.
Download figure:
Standard image High-resolution imageJudging from the XRD profiles, the hot-injection method produced single-phase cubic CsPb(Br1−x Ix )3 NCs. All of the peaks shifted to lower values of 2θ as the iodide ratio increased, indicating substitution of I− for Br− in CsPb(Br1−x Ix )3. The coordination number of the halide ion in cubic CsPb(Br1−x Ix )3 is six. 18 Given that Shannon's ionic radii of Br− and I− are 1.96 Å and 2.20 Å, respectively, 19 the lattice constant of cubic CsPbI3 (12.660 Å) is therefore larger than that of cubic CsPbBr3 (11.880 Å). 20 On the contrary, the NCs prepared by the ion-exchange methods contained a small amount of orthorhombic phase at higher iodide ratio, x. The cubic phase of CsPbI3 transforms to the more stable orthorhombic phase at room temperature. 21 Accordingly, the partial transition of cubic phase to orthorhombic phase at higher x might be attributed to the higher stability of the latter phase. 22
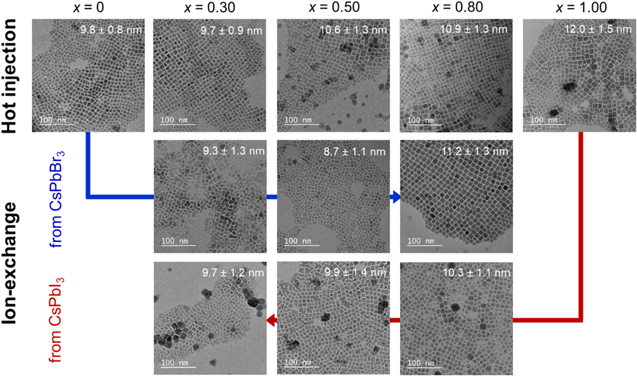
Judging from TEM images (Fig. 3), the average primary sizes of the prepared NCs were ∼10 nm. Most of the observed CsPbBr3 NCs and CsPbI3 NCs were smaller than their calculated exciton Bohr diameters of 7 nm and 12 nm, respectively. 15 The primary nanoparticles tended to become larger with increasing x because of increased lattice constant.
Figure 3. TEM images of CsPb(Br1−x Ix )3 NCs prepared by hot-injection method and ion-exchange methods.
Download figure:
Standard image High-resolution imageInvestigation of photoluminescence properties
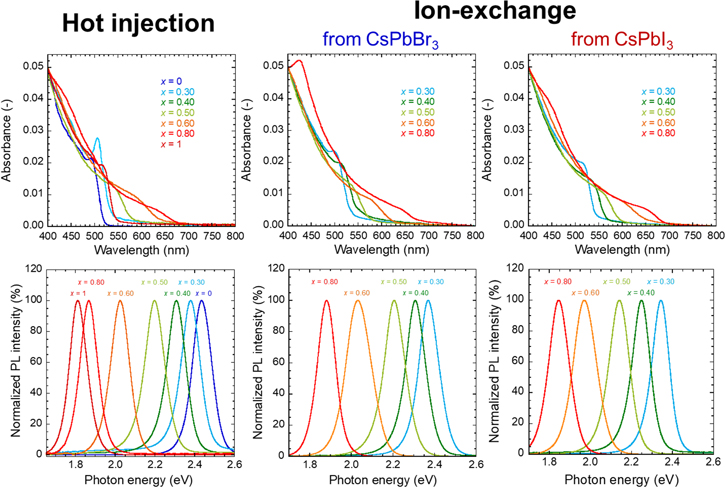
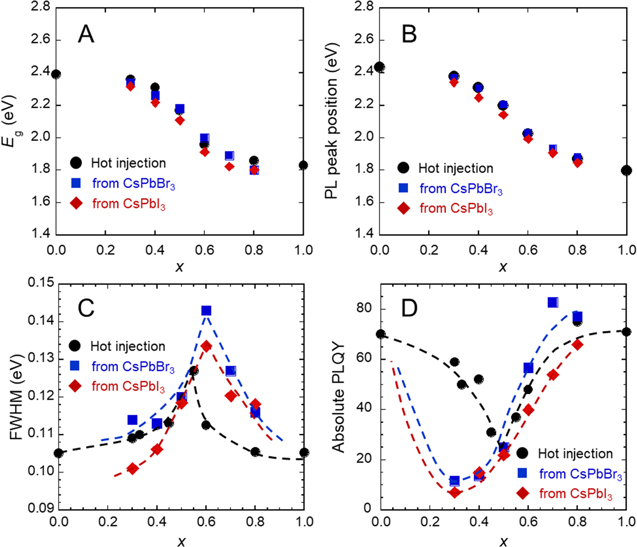
UV–vis absorption spectra and PL spectra are shown in Fig. 4. The absorption edge and emission peak position were shifted to longer wavelength and lower energy, respectively, by decreased bandgap because of the increased iodide ratio. 10 Bandgaps estimated from the UV–vis spectra and PL peak positions are summarized in Figs. 5A, 5B. Their behaviors overlapped well, regardless of the preparation methods, and no linear relationship was observed. This might be attributed to complex factors determining the bandgap, such as particle size and exciton Bohr diameter.
Figure 4. UV–vis spectra and PL spectra of CsPb(Br1−x Ix )3 NCs prepared by hot-injection method and ion-exchange methods.
Download figure:
Standard image High-resolution imageFigure 5. Changes in (A) bandgap, (B) PL peak position, (C) FWHM of PL peak, and (D) absolute PLQY of CsPb(Br1−x Ix )3 NCs prepared by hot-injection method and ion-exchange methods.
Download figure:
Standard image High-resolution imageFigure 5C shows the change in FWHM of the PL peak against the iodide ratio. The FWHM for NCs prepared by hot injection achieved a maximum at x = 0.55. In other words, the PL peak width of mixed-halide NCs at x = 0.55 was broader than those of CsPbBr3 and CsPbI3. This phenomenon might be attributed to the compositional inhomogeneity among the individual CsPb(Br1−x Ix )3 NCs. 8 However, the maximum occurred at larger x for NCs prepared by the ion-exchange methods. As mentioned above, a small amount of orthorhombic phase was observed for the NCs at higher x. Partial transition of orthorhombic phase from cubic phase for iodide-rich CsPb(Br1−x Ix )3 NCs might result in increased FWHM at higher x. PL decay times estimated from the PL decay curves shown in Fig. 6 were summarized in Table II. PL decay times of the CsPb(Br1−x Ix )3 NCs prepared at x = 0.50 were between those of CsPbBr3 NCs and CsPbI3 NCs regardless of the preparation method.
Figure 6. PL decay curves of CsPb(Br1−x Ix )3 NCs prepared by hot-injection method (x = 0, 0.50, and 1.00) and ion-exchange methods (x = 0.50).
Download figure:
Standard image High-resolution imageTable II. Summary of PL decay times of CsPb(Br1−x Ix )3 NCs prepared by hot-injection method (x = 0, 0.50, and 1.00) and ion-exchange methods (x = 0.50).
| x | Average (ns) 〈τ〉 | PL decay time (ns) | Amplitude (%) | ||||
|---|---|---|---|---|---|---|---|
| τ1 | τ2 | A1 | A2 | ||||
| Hot-injection | 0 | 4.6 | 2.4 | 7.6 | 81.7 | 18.3 | |
| 0.50 | 17.0 | 5.7 | 24.2 | 72.8 | 27.2 | ||
| 1.00 | 43.9 | 11.5 | 51.0 | 49.3 | 50.7 | ||
| Ion-exchange | from CsPbBr3 | 0.50 | 13.6 | 4.3 | 18.0 | 66.4 | 33.6 |
| from CsPbI3 | 0.50 | 20.9 | 5.7 | 32.2 | 80.9 | 19.1 | |
Change in PLQY with x is shown in Fig. 5D. The minimum PLQY for the hot-injection method was 25.0% at x = 0.50, which was smaller than PLQYs of CsPbBr3 (70%) and CsPbI3 (71.0%). The corresponding x value for the minimum PLQY was close to that for the maximum FWHM of the PL peak. The decreased PLQY of mixed-halide NCs could be attributed to lattice distortion induced by the difference in the ion radii of Br− and I−. 8 In contrast, the minimum PLQYs for halide exchanges from CsPbBr3 and CsPbI3 were likely to be located at x = ∼0.3, as described by the dashed lines in Fig. 5D. Furthermore, the minimum PLQYs for both halide exchanges were ∼10%, which was lower than 25.0% observed for the hot-injection method. PLQY is readily affected by internal defects in the NC and surface defects that cause nonradiative relaxation. The addition of a halide solution for ion-exchange might affect the adsorption state of OA and OAm surface modifiers, leading to the decreased PLQY.
Conclusions
This study compared the hot-injection method with the ion-exchange methods in terms of structure, morphology, and PL properties of CsPb(Br1−x Ix )3 NCs. There were no remarkable differences in properties for the NCs prepared from CsPbBr3 NCs and CsPbI3 NCs by ion-exchanges. The hot-injection method produced single-phase cubic CsPb(Br1−x Ix )3 NCs at every iodide ratio; however, the NCs prepared by ion exchange at higher x had a small amount of orthorhombic CsPb(Br1−x Ix )3. Primary particle size, bandgap, and PL peak position depended on halide composition regardless of the preparation method. FWHM of the PL peak and PLQY showed the maximum and minimum values, respectively, as the iodide ratio increased; however, their halide compositions were different for both preparation methods. For a deeper understanding of these phenomena, more versatile analyses will be required.
Acknowledgments
The authors are grateful for the financial support provided by the Futaba Research Grant Program of the Futaba Foundation (T.I.), the Iketani Science and Technology Foundation (T.I.), and the Shorai Foundation for Science and Technology (Y.I.).