Abstract
We have investigated the photoelectrochemical influence of quasi-solid-state electrolytes based on tailored ZnO nanostructures and mesoporous carbon electrocatalyst in the solar conversion performance of dye-sensitized solar cells (DSSCs). Tailored ZnO nanostructures with rod-shaped petals (ZnO nanorod) and almond-shaped petals (ZnO nanoalmond), were prepared using the hydrothermal method, respectively. Mesoporous carbon electrocatalyst with a high surface area is obtained by the carbonization of the PVDC-g-POEM double comb copolymer. The phase and structure of tailored ZnO nanostructures were investigated using scanning electron microscopy (SEM) and wide angle X-ray scattering (WAXS). Diffuse reflectance, intensity-modulated photocurrent spectroscopy (IMPS)/intensity-modulated photovoltage spectroscopy (IMVS), electrochemical impedance spectroscopy (EIS) measurements were used to investigate the optical and electrochemical properties of quasi-solid-state electrolytes. The quasi-solid-state electrolytes based on ZnO nanorod significantly improved the solar conversion performance owing to its enhanced scattering effect, ion diffusion, effective path for redox couple transfer, and sufficient penetration of quasi-solid-state electrolytes into the electrode. The quasi-solid-state electrolytes based on ZnO nanorod, ZnO nanoalmond and mesoporous carbon electrocatalyst showed a solar conversion efficiency of 6.4%, 5.4%, respectively, which is higher than that of the polymer gel electrolytes (4.7%).
Export citation and abstract BibTeX RIS
Dye-sensitized solar cells (DSSCs) composing mesoporous metal oxide-based photoanode, metal-based counter electrode, and liquid state electrolyte have reveled considerable attention over the last decade due to their advantage of high photoelectrochemical performance, low production cost and simple processing methods. 1,2 Yet, the use of liquid electrolyte in DSSCs has reviled a few limitations related to the leakage and volatilization of its solvent, which is known to lower the photoelectrochemical performance with time. To overcome, a number of research and discussions has been focused to seek for an ideal electrolyte with improved thermal and chemical stability, lower absorption coefficient in the visible region, less leakage and volatilization, and high ion conductivity and diffusivity in DSSCs. 3–6
With the advantages of thermal and chemical stability and less leakage and evaporation of solvent, several research has introduced different type of quasi solid-state electrolytes with poly(ethylene oxide) (PEO), poly(ethylene glycol) (PEG), or poly(methyl methacrylate) (PMMA) for DSSCs. 7–13 However, due to the limited ion diffusivity and permeability into mesoporous photoanodes, a relatively lower photoelectrochemical performance has been reported for DSSCs with quasi solid-state electrolytes, compared to that of liquid state-electrolyte. Thus, research on a non-liquid base electrolyte (quasi solid-state or solid-state) with a high conductivity and good interfacial contact to photo and counter electrodes has become very critical.
Shape control of the nanomaterials that serves as nanofillers into the solid-state electrolyte is being introduced to improve the photoelectrochemical performances of DSSCs. Through its simple and straight forward method, this is known to reduce the crystallinity of the polymer main chain and increase the ionic conductivity of the electrolyte by increasing free volume for redox couple transport. Zinc oxide is a semiconducting material with excellent stability in chemically that has been used for various applications including solar cells. Various types of ZnO nanostructures have gained attention due to their high-sensitivity, large specific area, non-toxicity, good compatibility and high isoelectric point. ZnO nanostructures are also easy to be tailored and designed into different size, structure, porosity and morphological features for different applications. However, compared to other metal oxides that are commonly selected as nanofiller materials due to the easiness of controlling their morphology, less has been discussed for ZnO as nanofiller material for mesoporous carbon electrocatalyst of DSSCs. 14–22
Mesoporous carbon materials are being realized as one of the strong candidates to replace the current precious-metal-based as counter electrode of DSSCs. Due to its high electrocatalytic activity, good electrochemical stability, and a large surface area for rapid interface reactions, mesoporous carbon materials are being advantageous for enhancing the overall performance. 23,24 Moreover, mesoporous carbon nanostructure can be directly formed from carbon precursors through a self-assembly of a block copolymer. 25 However, research of implementing double comb copolymer as the mesoporous carbon counter electrode in DSSCs is still considered to be in their preliminarily stage, where more gaps need to be filled in.
In this study, effect of the shape-controlled ZnO nanomaterials for quasi-solid-state electrolytes has been systematically investigated through the photoelectrochemical characteristics of the mesoporous carbon based DSSCs. As a nanofiller material, different shape of ZnO nanostructures (ZnO almond, ZnO nanorod) was tailored through hydrothermal method with different reaction condition. Respective ZnO almond and ZnO nanorod were selectively added to the quasi-solid-state electrolytes to improve the photochemical performance of the DSSCs. In order to obtain a mesoporous carbon electrocatalyst with higher surface area for counterelectrode, PVDC-g-POEM double comb copolymer has been carbonized. Finally, the effect of different ZnO nanostructures in quasi-solid-state electrolytes for mesoporous carbon based DSSCs was reported through a comparison research to that of polymer gel electrolytes.
Experimental
Materials
PEG (Mw = 10000 g mol−1), 1-methyl-3-propylimidazolium iodide (MPII), lithium iodide (LiI), lodine (I2), titanium(IV) bis(ethyl acetoacetato) diisopropoxide, zinc nitrate hexahydrate, hexamethylenetetramine (HMTA), polyethylenimine (PEI; Mw = 800 g mol−1), chloroplatinic acid hexahydrate (H2PtCl6), and di-tetrabutylammonium cis-bis(isothiocyanato) bis (2,2'-bipyridyl-4,4'-dicarboxylato)ruthenium(II) (N719) were obtained from Sigma Aldrich (St. Louis, Mo. USA). Acetonitrile, ethanol, 1-buthanol, 2-propanol, acetone, and tetrahydrofufan (THF) were obtained from J. T. Baker (Thermo Fisher Scientific, Waltham, MA, USA). Titania paste and conductive fluorin-doped tin oxide (FTO; TEC 7) was obtained from Dyesol (Aubonne, Switzerland) and Pilkington (France), respectively. Deionized water (>18 MΩm) was obtained with a water purification system made by MilliporeSigma (Burlington, MA, USA). All solvents and chemical reagents were obtained from commercial sources as guaranteed grade reagents. They were used in the experiments without further purification.
Preparation of tailored ZnO nanostrucrues
The two types of tailored ZnO nanostructures (ZnO almond, ZnO nanorod) were synthesized via facile hydrothermal method. First, the tailored ZnO nanostructures seed solution was prepared by zinc nitrate hydrate (0.050 M), HMTA (0.050 M), PEI (0.005 M) in ammonium hydroxide (0.35 M) and stirring for 1 h at room temperature. FTO glass was cleaned with chloroform and 2-propanol and placed in a Teflon-lined autoclave, wherein the conductive side of the FTO was placed face down. Then, as prepared the two types of tailored ZnO nanostructures (ZnO nanoalmond, ZnO nanorod) solution was transferred into the Teflon-lined autoclave followed by heating at 90 °C for 12, 15 h, respectively. And then, the two types of tailored ZnO nanostructures (ZnO nanoalmond, ZnO nanorod) were collected from each powder after being washed with DI-water several times and dried at 50 °C in the air for a day.
Preparation of tailored ZnO quasi-solid-state electrolyte
Two types of tailored ZnO quasi-solid-state electrolytes were prepared for the fabrication of dye-sensitized solar cells (DSSCs): (i) a ZnO nanoalmond quasi-solid-state electrolytes consisting of ZnO nanoalmond, PEG (Mn = 400 g mol−1), LiI, MPII, and I2 in acetonitrile; ii) a ZnO nanorod quasi-solid-state electrolytes consisting of ZnO nanorod, PEG (Mn = 400 g mol−1), LiI, MPII, and I2 in acetonitrile, respectively. The mole ratio of ether oxygen to iodide salt was fixed at 20, the iodine content was fixed at 10 wt% to the salt, and the tailored ZnO nanostructures content was fixed at 10 wt% to PEG. In addition, polymer gel electrolytes were prepared according to a previous report as control samples. 19
Preparation of mesoporous carbon electrocatalyst based counterelectrode
The mesoporous carbon electrocatalyst-based counter electrode was prepared by depositing on the prepared FTO substrate according to the modified protocol previously reported by our group. 26 In brief, 0.05 g of PVDC-g-POEM double comb copolymer was first dissolved in 1.5 ml of THF. Separately, 0.15 ml of concentrated HCl (37 wt%) was slowly added to 0.15 ml of deionized water at room temperature under vigorous stirring. After aging for 15 min at room temperature, the HCl/H2O solution was slowly added to the PVDC-g-POEM double comb copolymer solution while stirring, followed by annealing at 60 °C for 1 h to obtain a viscous solution. Then, the viscous PVDC-g-POEM double comb copolymer solution was screen-printed on the FTO substrate, and the printed film was calcinated at 550 °C in Ar for 30 min.
Preparation of tailored ZnO quasi-solid-state electrolyte based DSSCs
The FTO substrate was cleaned with chloroform and 2-propanol. As a photoanode, the cleaned FTO conductive side was spin coated with 1 wt% titanium diisopropoxide bis(ethylacetoacetate) 2-butanol solution followed by calcination at 450 °C for 30 min. To prepare nanocrystalline TiO2, commercial TiO2 paste (Ti-Nanoxide, D20) was deposited on the prepared FTO substrate using the doctor-blade method and dried at 50 °C and 80 °C for 1 h, respectively, followed by sintering at 450 °C for 30 min. Then, the prepared photoanode was immersed in 0.1 mM N719 dye ethanol solution at 50 °C for 3 h. According to a previously reported procedure, DSSCs with an active area of 0.4 cm2 were constructed by drop-casting of tailored ZnO quasi-solid-state electrolytes solution onto the photoanode and covering with the counterelectrode. 27–29 And, then the DSSCs were dried in a drying oven for 24 h for solvent evaporation.
Characterization
The morphologies were acquired by field emission scanning electron microscopy (FE-SEM) (SU8010, Hitachi, Tokyo, Japan). The crystalline structure of tailored ZnO was obtained by X-ray diffraction (XRD) spectroscopy (Smartlab, Rigaku, Tokyo, Japan) with Cu–Kα radiation (λ = 1.5406 Å). UV–vis diffuse-reflectance spectra were characterized by spectrophotometry (Mega-900, Scinco, Seoul, South Korea). The specific surface area and pore volume of the mesoporous carbon electrocatalysts were measured using the N2 adsorption-desorption isotherm via the Brunauer–Emmett–Teller (BET) methods. Before the adsorption-desorption measurement, the mesoporous carbon was degassed at 70 °C under a dynamic vacuum (10−2 Torr) for 1 h. Intensity-modulated photocurrent/voltage spectroscopy (IMPS/IMVS) was used to investigate electron diffusion and recombination properties. Thickness of photoanode and counterelectrode was measured using an alpha-step IQ surface profile (KLA Tencor). The current density–voltage (J–V) measurements were performed using a potentiostat (Compactstat.h, Ivium Technologies, Eindhoven, The Netherlands). The solar simulator equipped with a 1000 W xenon lamp was used as light source (SunLite, Abet Technologies, Inc., Milford, CT, USA). Electrochemical impedance spectroscopy (EIS) characteristics were measured by a potentiostat under open-circuit voltage in a frequency range of 0.01 Hz–100 kHz. Incident photo-to-electron conversion efficiency (IPCE) spectra were obtained by IPCE system (IPCEPEIPCE100S, HS Technologies, Plano, TX, USA). The photovoltaic parameters were calculated using Eqs. 1 and 2:
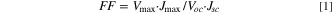
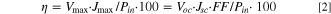
where η is the photovoltaic conversion efficiency, Jsc is short-circuit current density, Voc is the open-circuit voltage, Pin is incident light power, FF is fill factor, and Jmax and Vmax are current density and voltage, respectively, when maximum power is achieved.
Results and Discussion
DSSCs fabricated with different electrolyte of (a) polymer gel (b) ZnO nanoalmond quasi-solid-state, and (c) ZnO nanorod quasi-solid-state is shown in Scheme
Scheme 1. The schematic illustration of the mesoporous carbon electrocatalyst based DSSCs fabricated with (a) polymer gel electrolyte, (b) ZnO nanoalmond quasi-solid-state electrolyte, and (c) ZnO nanorod quasi-solid-state electrolyte.
Download figure:
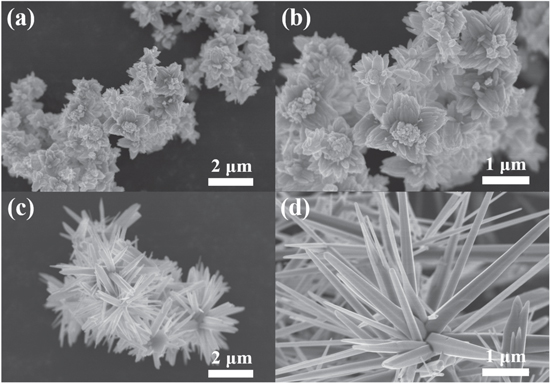
Standard image High-resolution imageFigure 1 shows the FE-SEM images of the ZnO structures (ZnO nanoalmond and ZnO nanorod) that were obtained through hydrothermal reaction with different reaction time of 12 h and 15 h, respectively. Both ZnO nanostructures were grown into a radial shape where the core of the homogeneous structure reaching towards the outside in a circular direction. Still, ZnO nanoalmond (a,b) and ZnO nanorods (c,d) were significantly distinguishable to one another by their shape, length and width of the nanostructure. ZnO nanoalmond shows a shape of multi layered almonds in its lengthwise direction that is similar to an oval shape with a sharpen vertex. Also, a number of flower-like shape was found at each center of the multi layered almond structures, similar to that of a daffodil. Lengths of each ZnO nanoalmond were found between 0.3 μm and 1 μm while the widths were found between 0.1 μm and 0.8 μm. ZnO nanorods showed a shape of multiple blades arranged in a radial form, heading towards outer circle from the center. Lengths of each nanorods were found between 0.9 μm and 3 μm, while the widths were between 0.1 μm and 0.8 μm. The structural difference of ZnO nanoalmonds and nanorods has been realized by the different reaction time of ZnO solution during the hydrothermal growth condition inside the autoclave.
Figure 1. FE-SEM image of the (a) ZnO nanoalmond, (b) magnified ZnO nanoalmond, (c) ZnO nanorod and (d) magnified ZnO nanorod.
Download figure:
Standard image High-resolution imageFigure 2a shows (a) the XRD patterns of ZnO nanalmonds and ZnO nanorod. Sharp peaks at 2 thata values of 31.84°, 34.52°, 36.33°, 47.63°, 56.71°, 62.96°, 68.13°, and 69.18° have been identically found in both ZnO nanostructures with lattice constants a = b = 0. Each peak corresponds to (100), (002), (101), (102), (110), (103), (200), (112), and (201) planes of wurtzite hexagonal crystalline phase (JCPDS file no. 36-1451). 32 This also indicates that the preparation of tailored ZnO nanostrucrues (ZnO nanoalmonds and ZnO nanorod) using the hydrothermal process has been successful. Figure 2b shows the scattering effect of each electrolytes that was characterized by UV–vis diffuse reflectance spectra. At short wavelength region between 300 nm and 550 nm, the diffuse reflectance value of all three electrodes was found at a very similar range. The sequence of diffuse reflectance showed ZnO nanorod > ZnO nanoalmond > polymer gel, while the maximum difference of each electrolytes were below 1.5%. However, at the wavelength between 550 nm and 740 nm, the difference in diffuse reflectance between ZnO nanorod quasi-solid-state electrolyte and two other electrolytes has gradually increased. At 740 nm, the diffusion reflectance of ZnO nanorods (13%) showed the approximately twice the amount of that of ZnO nanoalmond (7%) and polymer gel (6%). This comparably higher diffusion reflectance with a steep increasement in a particular wavelength indicates that the use of ZnO nanorods in quasi-solid-state electrolyte has enhanced scattering effect, which is advantageous for leading a higher light-harvesting property of DSSCs.
Figure 2. (a) XRD patterns of ZnO nanoalmond and ZnO nanorod, (b) UV–vis diffuse reflectance spectra as Kubelka-Munk (KM) function of polymer gel electrolyte, ZnO nanoalmond quasi-solid-state electrolyte, and ZnO nanorod quasi-solid-state electrolyte.
Download figure:
Standard image High-resolution imageFigure 3 shows the N2 adsorption–desorption isotherm of mesoporous carbon electrocatalyst derived from PVDC-g-POEM double comb copolymer. Results of BET surface area (472.15 m2 g−1) and specific pore volume (0.74 cm3 g−1) indicates the successful formation of highly porous carbon material with well-defined mesoporous structure was carried through a self-assembled double comb polymer template carbon source. 26 Also, the mesoporous carbon electrocatalyst derived from PVDC-g-POEM double comb copolymer also exhibited a Type IV N2 isotherm where the hysteresis loop approached relatively higher pressures, indicating multilayer adsorption followed by capillary condensation.
Figure 3. (a) N2 adsorption (filled symbols) and desorption (unfilled symbols) isotherms of mesoporous carbon electrocatalyst derived from PVDC-g-POEM double comb copolymer. (b) SEM of mesoporous carbon electrocatalyst.
Download figure:
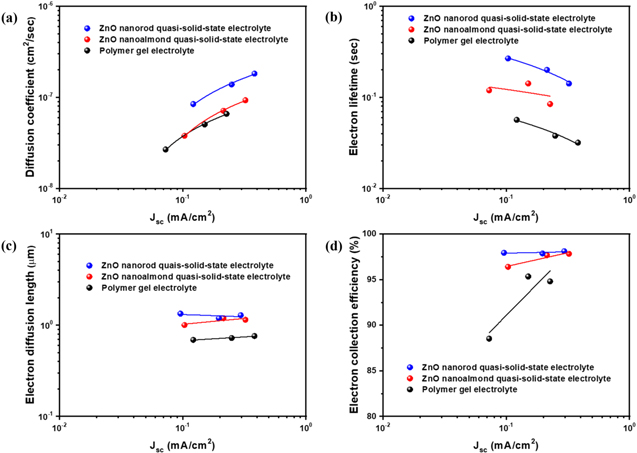
Standard image High-resolution imageFigure 4 shows the results of electron transport properties of three different types of electrolyte through diffusion coefficient (Dn), electron lifetime (τr), electron diffusion length (Ln), and electron collection efficiency (ηcc). As a function of Jsc , ηcc was characterized using IMPS/IMVS analysis to evaluate electron transport time (τt ) and electron lifetime (τr ), which the τt being converted to diffusion coefficient (Dn) as below:
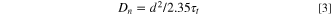
while d represents the film thickness on FTO photoanode. Equations 4 and 5 was used to obtain electron diffusion length (Ln), and electron collection efficiency (ηcc) as below:
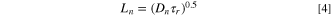
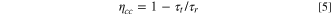
with τr and τt values being calculated by IMVS and IMPS analysis, respectively. 33,34 Compared to polymer gel electrolyte, higher values of Dn and τr of DSSCs was achieved for both ZnO nanostructure (nanoalmond and nanorod) quasi-solid-state electrolyte. This further indicats that the electron transfer of photoelectrochemical devices has been enhanced by using ZnO as nanofiller in electrolyte. Moreover, both quasi-solid-state electrolyte with ZnO nanostructure (ZnO nanoalmond and ZnO nanorod) showed a higher Ln and ηcc values compared to polymer gel electrolyte at all ranges of Jsc. This indicates that the facile formation of amorphous region of polymer part in ZnO nanstructure quasi-solid-state electrolyte has provided an effective path for redox couple transfer, which has successfully led the enhanced the charge transport while reducing the recombination of the photoelectrochemical devices. Also, micrometer-sized inorganic nanofiller in ZnO nanostructure quasi-solid-state electrolyte could potentially lead to enhanced scattering effect on the interface of electrolyte/photoanode. It should be noted that similar observations have previously been reported regarding the influence of the micrometer-sized inorganic nanofiller on the electron transfer properties. 14
Figure 4. (a) Diffusion coefficients (Dn ), (b) recombination time (τr ), (c) electron diffusion length (Ln), and (d) electron collection efficiency (ηcc) as functions of the Jsc for the mesoporous carbon electrocatalyst based dye-sensitized solar cells (DSSCs) with polymer gel electrolyte, ZnO nanoalmond quasi-solid-state electrolyte, and ZnO nanorod quasi-solid-state electrolyte measured by IMPS and IMVS.
Download figure:
Standard image High-resolution imagePhotovoltaic properties of mesoporous carbon based DSSCs with different electrolyte are shown in Fig. 5a through (a) J-V curves. Respective data of each photovoltaic parameters of short-circuit current density (Jsc), open-circuit voltage (Voc), FF, and overall energy conversion efficiency (η) are listed in Table I. Jsc, Voc, FF, and η value of mesoporous carbon based DSSCs with polymer gel electrolyte has shown 0.72 V, 10.0 mA cm−2, 0.64, and 4.7% that is comparably lower than those values collected from ZnO nanstructure quasi-solid-state electrolyte based to other types of DSSCs. ZnO nanorods quasi-solid-state electrolyte has achieved the highest achieved values of 0.77 V, 11.4 mA cm−2, 0.75 and 6.4%, which followed by ZnO nanoalmond based DSSC with values of 0.75 V, 10.9 mA cm−2, 0.73, and 5.4%. The highest efficiency (6.4%) of DSSC achieved in this research by using ZnO nanorod quasi-solid-state electrolyte and mesoporous carbon electrocatalyst is also identified as the one of the highest values among other type of Pt-free counter electrode based DSSCs to date. 35–39 Moreover, the Jsc of DSSC with ZnO quasi-solid-state electrolyte reports each 9% (ZnO nanoalmond) and 14% (ZnO nanorod) higher compared to that of DSSCs with polymer gel electrolyte. These results indicates that the introduction of ZnO to quasi-solid-state electrolyte has improved the light-harvesting effect of DSSCs by the enhancement in both ion diffusion of the redox couple and the scattering effect. Interestingly, the Voc value also increased up to 0.77 V using the ZnO nanorod quasi-solid-state electrolyte, which resulted from the decreased charge recombination, electron back reaction loss in mesoporous carbon based DSSCs. Figure 5b shows the equivalent circuit diagram of the mesoporous carbon based DSSCs with ZnO nanostructure quasi-solid-state electrolyte, while the corresponding electrochemical parameters being listed in Table II. Equivalent circuit model includes the series resistance (Rs ) that is the ohmic resistance of the FTO substrate, interfacial charge transfer resistance between counter electrode/electrolyte (R1), interfacial charge transfer resistance between photoanode/electrolyte (R2), and Warburg diffusion resistance (Ws ). While matching the equivalent circuit with the Nyquist impedance data at Fig. 5c, a significant decrease in the total resistance of mesoporous carbon based DSSCs with ZnO nanstructure quasi-solid-state electrolyte was found, compared to that of polymer gel. This can be due to the increase of electron transport in mesoporous carbon based DSSCs with ZnO nanostructure quasi-solid-state electrolyte that may lead to the decrease of resistance at the photoanode/electrolyte interface and enhanced FF values. Incident photon-to-electron conversion efficiency (IPCE) spectra in Fig. 5d demonstrates the light-harvesting efficiencies and the internal quantum of mesoporous carbon based DSSCs with ZnO nanostructure quasi-solid-state electrolyte at an incident wavelength. Maximum IPCE value of 70% and 68% was respectively found at the DSSCs with ZnO nanorod and ZnO nanoalmond quasi-solid-state electrolytes, which is comparably higher to that of DSSCs based on the polymer gel electrolyte (63%). Also, the IPCE spectra were integrated in order to calculate the photocurrent density gained and were compared with the actual photocurrents obtained by J-V measurements. As shown in Table I, the photocurrent density values from the J-V curves were well consistent with those determined from IPCE curves for for every types of mesoporous carbon based DSSCs. Improved IPCE value of the mesoporous carbon based DSSCs with the ZnO nanostructure quasi-solid-state electrolyte was due to the improved light harvesting efficiency that is consistent to the previous diffuse reflectance.
Figure 5. (a) J-V curves, (b) equivalent circuit diagram, (c) EIS Nyquist plots, and (d) IPCE curves of mesoporous carbon electrocatalyst based DSSCs fabricated with polymer gel electrolyte, ZnO nanoalmond quasi-solid-state electrolyte, and ZnO nanorod quasi-solid-state electrolyte at 100 mW cm−2.
Download figure:
Standard image High-resolution imageTable I. Photovoltaic properties of mesoporous carbon electrocatalyst based dye-sensitized solar cells (DSSCs) with polymer gel electrolyte, ZnO nanoalmond quasi-solid-state electrolyte, and ZnO nanorod quasi-solid-state electrolyte at 100 mW cm−2 (AM 1.5). a),b),c),d),e),f),g),h)
| Electrolyte | Voc (V) | Jsc g) (mA cm−12) | Jsc h) (mA cm−12) | FF | η (%) |
|---|---|---|---|---|---|
| Polymer gel electrolyte | 0.72 | 10.0 | 9.8 | 0.64 | 4.7 |
| ZnO nanoalmond quasi-solid-state electrolyte | 0.75 | 10.9 | 10.5 | 0.73 | 5.4 |
| ZnO nanorod quasi-solid-state electrolyte | 0.77 | 11.4 | 11.1 | 0.75 | 6.4 |
a)Polymer gel electrolyte consists of PEG, LiI, MPII, and I2 in acetonitrile. b)ZnO nanoalmond quasi-solid-state electrolyte consist of commercial ZnO nanoalmond, PEG, LiI, MPII, and I2 in acetonitrile. c)ZnO nanorod quasi-solid-state electrolyte consist of commercial ZnO nanorod, PEG, LiI, MPII, and I2 in acetonitrile. d)The photoactive area for DSSCs was 0.16 cm2. e)The thickness of the photoanode was approximately 7 μm. f)The thickness of the counterelectrode was approximately 5 μm. g)Determined from J-V curves. h)Determined from IPCE curves.
Table II. Electrochemical parameters of mesoporous carbon electrocatalyst based DSSCs with polymer gel electrolyte, ZnO nanoalmond quasi-solid-state electrolyte, and ZnO nanorod quasi-solid-state electrolyte, as determined by EIS analysis at 100 mW cm−2 (AM 1.5).
| Electrolyte | RS (Ω) | R1 (Ω) | R2 (Ω) | WS (Ω) |
|---|---|---|---|---|
| Polymer gel electrolyte | 12.9 | 5.4 | 32.5 | 1.2 |
| ZnO nanoalmond quasi-solid-state electrolyte | 12.4 | 6.1 | 26.8 | 0.7 |
| ZnO nanorod quasi-solid-state electrolyte | 12.8 | 5.6 | 24.8 | 0.5 |
Conclusions
For improved photoelectrochemical performance of mesoporous carbon based DSSCs, ZnO nanomaterials were prepared as a nanofiller in a PEG-based quasi-solid-state electrolyte to enhance ion diffusion, scattering effect, effective path for redox couple transfer, and sufficient penetration of quasi-solid-state electrolyte. Two types of ZnO nanomaterials of ZnO nanoalmond and ZnO nanorods were prepared by hydrothermal method with respective reaction hours. Mesoporous carbon electrocatalyst on the FTO counterelectrode substrate was prepared through the carbonization of PVDC-g-POEM double comb copolymers. The mesoporous carbon based DSSCs based on ZnO nanorods quasi-solid-state electrolyte (6.4%) showed a higher cell performance than that with ZnO nanoalmond quasi-solid-state electrolyte (5.4%), and polymer gel that was prepared as a reference of quasi solid-state electrolyte (4.7%). By adding ZnO nanofiller to a quasi-solid-state electrolyte, significant improvement has been achieved in the light-harvesting, charge transport, reduces recombination, and decreases the resistance at the electrode/electrolyte interface of DSSCs. We believe that a promising method of fabricating a mesoporous carbon based photoelectrochemical application with enhanced performance has been successfully presented by introducing shape controlled ZnO nanostructures and mesoporous carbon electrocatalyst.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2019R1C1C1010283). This paper was written as part of Konkuk University's research support program for its faculty on sabbatical leave in 2021.