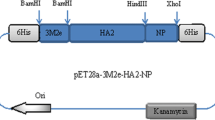
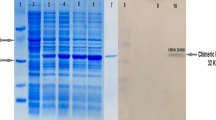
Abstract
Purpose
Influenza is one of the most important agents of pandemic outbreak causing substantial morbidity and mortality. Vaccination strategies of influenza must be adapted annually due to constant antigenic changes in various strains. Therefore, the present study was conducted to evaluate protective immunity of the conserved influenza proteins.
Methods
For this purpose, three tandem repeats of M2e (3M2e) and NP were separately expressed in E. coli and were purified using column chromatography. Female Balb/c mice were injected intradermally with a combination of the purified 3M2e and NP alone or formulated with Alum (AlOH3) adjuvant in three doses. The mice were challenged by intranasal administration of H1N1 (A/PR/8/34) 2 weeks after the last vaccination.
Results
The results demonstrated that recombinant NP and M2e proteins are immunogenic and could efficiently elicit immune responses in mice compared to non-immunized mice. The combination of 3M2e and NP supplemented with Alum stimulated both NP and M2e-specific antibodies, which were higher than those stimulated by each single antigen plus Alum. In addition, the secretion of IFN-γ and IL-4 as well as the induction of lymphocyte proliferation in mice received the mixture of these proteins with Alum was considerably higher than other groups. Moreover, the highest survival rate (86%) with the least body weight change was observed in the mice immunized with 3M2e and NP supplemented with Alum followed by the mice received NP supplemented with Alum (71%).
Conclusion
Accordingly, this regimen can be considered as an attractive candidate for global vaccination against influenza.





Similar content being viewed by others
Data availability
All data are available in case of need.
Abbreviations
- 3M2e:
-
Three tandem repeats of M2e
- M2e:
-
M2 extracellular domain
- HA:
-
Hemagglutinin
- NA:
-
Neuraminidase
- UIV:
-
Universal influenza vaccines
- NP:
-
Nucleoprotein
- IAV:
-
Influenza A virus
- rNP:
-
Recombinant nucleoprotein
- Th2:
-
T Helper 2
- IgG1:
-
Immunoglobulin G1
- IgE:
-
Immunoglobulin E
- SDS:
-
Acrylamide and sodium dodecyl sulphate
- IPTG:
-
Isopropyl β-d-1-thiogalactopyranoside
- TMB:
-
Tetramethylbenzidine
- 3-(4,5-dimethyl tetrazolyl-2) 2,5 diphenyl:
-
MTT salt
- HRP:
-
Horseradish peroxidase
- ELISA:
-
Enzyme-linked immunosorbent assay
- LEW:
-
Lysis-equilibration-wash
- PBS:
-
Phosphate buffer saline
- DMSO:
-
Dimethyl sulfoxide
- SI:
-
Stimulate index
- IFN-γ:
-
Interferon-γ
- IL-4:
-
Interleukin 4
- LD90 :
-
Lethal Dose 90
- APCs:
-
Antigen-presenting cells
- HSP70:
-
Heat shock proteins
- CTL:
-
Cytotoxic T lymphocyte
- LB Broth:
-
Luria-Bertani Broth
References
Awate S, Babiuk L, Mutwiri G (2013) Mechanisms of action of adjuvants. Front Immunol 4:114
Bernelin-Cottet C, Deloizy C, Stanek O, Barc C, Bouguyon E, Urien C, Da Costa B (2016) A universal influenza vaccine can lead to disease exacerbation or viral control depending on delivery strategies. Front Immunol 7:641
Bernstein ED, Gardner EM, Abrutyn E, Gross P, Murasko DM (1998) Cytokine production after influenza vaccination in a healthy elderly population. Vaccine 16:1722–1731
Carragher D, Kaminski D, Moquin A, Hartson L, Randall T (2008) A novel role for non-neutralizing antibodies against nucleoprotein in facilitating resistance to influenza virus. J Immunol Res 181:4168–4176
De Filette M, Fiers W, Martens W, Birkett A, Ramne A, Löwenadler B, Saelens X (2006a) Improved design and intranasal delivery of an M2e-based human influenza A vaccine. Vaccine 24:6597–6601
De Filette M, Ramne A, Birkett A, Lycke N, Löwenadler B, Jou W, Fiers W (2006b) The universal influenza vaccine M2e-HBc administered intranasally in combination with the adjuvant CTA1-DD provides complete protection. Vaccine 24:544–551
Ebrahimi S, Tebianian M (2011) Influenza A viruses: why focusing on M2e-based universal vaccines. Virus Genes 42:1–8
Eisenbarth S, Colegio O, O’Connor W, Sutterwala F, Flavell RA (2008) Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453:1122–1126
Farahmand B, Taheri N, Shokouhi H, Soleimanjahi H, Fotouhi F (2019) Chimeric protein consisting of 3M2e and HSP as a universal influenza vaccine candidate: from in silico analysis to preliminary evaluation. Virus Genes 55:22–32
Fiers W, De Filette M, El Bakkouri K, Schepens B, Roose K, Schotsaert M, Saelens X (2009) M2e-based universal influenza A vaccine. Vaccine 27:6280–6283
Gao X, Wang W, Zhang S, Duan Y, Xing L, Wang X (2013) Enhanced influenza VLP vaccines comprising matrix-2 ectodomain and nucleoprotein epitopes protects mice from lethal challenge. Antivir Res 98:4–11
Guo L, Zheng M, Ding Y, Yang Z, Wang H, Chen Z (2010) Protection against multiple influenza A virus subtypes by intranasal administration of recombinant nucleoprotein. Arch Virol 155:1765–1775
Huleatt J, Nakaar V, Desai P, Huang Y, Hewitt D, Jacobs A, Umlauf S (2008) Potent immunogenicity and efficacy of a universal influenza vaccine candidate comprising a recombinant fusion protein linking influenza M2e to the TLR5 ligand flagellin. Vaccine 26:201–214
Jordan M, Mills D, Kappler J, Marrack P, Cambier J (2004) Promotion of B cell immune responses via an alum-induced myeloid cell population. Science 304:1808–1810
Kaiser J (2006) A one-size-fits-all flu vaccine. Sience|AAAS 380–382
Kool M, Pétrilli V, De Smedt T, Rolaz A, Hammad H, Van Nimwegen M, Tschopp J (2008) Cutting edge: Alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol Res 181:3755–3759
Lamb R, Zebedee S, Richardson C (1985) Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell 40:627–633
Lee Y, Kim K, Ko E, Lee Y, Kim M, Kwon Y, Kang S (2014) New vaccines against influenza virus. Clin Exp Vaccine Res 3:12–28
Li Z, Gabbard J, Mooney A, Gao X, Chen Z, Place R, He B (2013) Single-dose vaccination of a recombinant parainfluenza virus 5 expressing NP from H5N1 virus provides broad immunity against influenza A viruses. J Virol 87:5985–5993
MacLeod M, David A, Jin N, Noges L, Wang J, Kappler J, Marrack P (2013) Influenza nucleoprotein delivered with aluminium salts protects mice from an influenza A virus that expresses an altered nucleoprotein sequence. PLoS ONE 8:e61775
Mardanova E, Kotlyarov R, Kuprianov V, Stepanova L, Tsybalova L, Lomonossoff G, Ravin N (2015) High immunogenicity of plant-produced influenza based on the M2e peptide fused to flagellin. Biotechnol Lett 15:25
Mbow M, De Gregorio E, Valiante N, Rappuoli R (2010) New adjuvants for human vaccines. Curr Opin Immunol 22:411–416
Morefield G, Sokolovska A, Jiang D, HogenEsch H, Robinson J, Hem S (2005) Role of aluminum-containing adjuvants in antigen internalization by dendritic cells in vitro. Vaccine 23:1588–1595
Pica N, Palese P (2013) Toward a universal influenza virus vaccine: prospects and challenges. Annu Rev Med 64:189–202
Rafati S, Gholami E, Hassani N, Ghaemimanesh F, Taslimi Y, Taheri T, Soong L (2007) Leishmania major heat shock protein 70 (HSP70) is not protective in murine models of cutaneous leishmaniasis and stimulates strong humoral responses in cutaneous and visceral leishmaniasis patients. Vaccine 25:4159–4169
Rappazzo C, Watkins H, Guarino C, Chau A, Lopez JL, DeLisa M, Putnam D (2016) Recombinant M2e outer membrane vesicle vaccines protect against lethal influenza A challenge in BALB/c mice. Vaccine 34:1252–1258
Reed S, Bertholet S, Coler R, Friede M (2009) New horizons in adjuvants for vaccine development. Trends Immunol 30:23–32
Saleh M, Nowroozi J, Farahmand B, Fotouhi F (2020) An approach to the influenza chimeric subunit vaccine (3M2e-HA2-NP) provides efficient protection against lethal virus challenge. Biotechnol Lett. https://doi.org/10.1007/s10529-020-02822-3
Shokouhi H, Farahmand B, Ghaemi A, Mazaheri V, Fotouhi F (2018) Vaccination with three tandem repeats of M2 extracellular domain fused to Leismania major HSP70 protects mice against influenza A virus challenge. Virus Res 251:40–46
Slepushkin VA, Katz JM, Black RA, Gamble WC, Rota PA, Cox NJ (1995) Protection of mice against influenza A virus challenge by vaccination with baculovirus-expressed M2 protein. Vaccine 13:1399–1402
Thomas P, Keating R, Hulse-Post D, Doherty P (2006) Cell-mediated protection in influenza infection. Emerg Infect Dis 12:48
Tompkins S, Zhao Z, Lo C, Misplon J, Liu T, Epstein S (2007) Matrix protein 2 vaccination and protection against influenza viruses, including subtype H5N1. Emerg Infect Dis 13:426
Wang W, Huang B, Jiang T, Wang X, Qi X, Gao Y, Ruan L (2012) Robust immunity and heterologous protection against influenza in mice elicited by a novel recombinant NP–M2e fusion protein expressed in E. coli. PLoS ONE 7:e52488
Wei J, Li Z, Yang Y, Ma X, An W, Ma G, Zhang S (2020) A biomimetic VLP influenza vaccine with interior NP/exterior M2e antigens constructed through a temperature shift-based encapsulation strategy. Vaccine 38:5987–5996
Yang P, Wang W, Gu H, Li Z, Zhang K, Wang Z, Wang X (2014) Protection against influenza H7N9 virus challenge with a recombinant NP–M1–HSP60 protein vaccine construct in BALB/c mice. Antivir Res 111:1–7
Yousefi-Najafabadi Z, Behzadian F, Fotouhi-Chahooki F, Farahmand B (2013) Prokaryotic expression of influenza A virus nucleoprotein fused to mycobacterial heat shock protein70. Iran J Virol 7:7–14
Zhao G et al (2010a) Induction of protection against divergent H5N1 influenza viruses using a recombinant fusion protein linking influenza M2e to Onchocerca volvulus activation associated protein-1 (ASP-1) adjuvant. Vaccine 28:7233–7240
Zhao G, Lin Y, Du L, Guan J, Sun S, Sui H, Zheng B (2010b) An M2e-based multiple antigenic peptide vaccine protects mice from lethal challenge with divergent H5N1 influenza viruses. Virol J 7:1–8
Zheng M, Luo J, Chen Z (2014) Development of universal influenza vaccines based on influenza virus M and NP genes. Infection 42:251–262
Acknowledgements
This study was performed in the Department of Influenza and Other Respiratory Viruses, Pasteur Institute of Iran and was also financially supported by the Pasteur Institute of Iran (Grant Number: 759). Hereby, the authors would like to show their gratitude to all the staff working in Pasteur Institute of Iran for their valuable assistance and cooperation.
Funding
This work was supported by the Pasteur Institute of Iran (Grant Number 759).
Author information
Authors and Affiliations
Contributions
(MS): Study concept and design; Development of the protocol; Acquisition, analysis and interpretation of data; Draft of the manuscript, wrote and prepared the manuscript; and Critical revision of the manuscript for important intellectual content. (JN): Study concept and design; Supervision; Administrative; Technical and material support. (BF): Study concept and design; Supervision; Administrative; Technical, and material support. (FF): Study concept and design; Development of the original idea and the protocol; Administrative; Technical, and material support; Supervision; and Interpretation of data.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interests.
Ethical approval
All the animal experiments were approved and performed based on the guidelines of the Ethical Committee of the Pasteur Institute of Iran, Tehran, Iran (IR.PII.REC.1395.82).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Forqani, M., Hosseini, S.M., Farahmand, B. et al. Combination of conserved recombinant proteins (NP & 3M2e) formulated with Alum protected BALB/c mice against influenza A/PR8/H1N1 virus challenge. Biotechnol Lett 43, 2137–2147 (2021). https://doi.org/10.1007/s10529-021-03174-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-021-03174-2