Abstract
To evaluate the effectiveness of the BNT162b2 messenger RNA vaccine in pregnant women, we conducted an observational cohort study of pregnant women aged 16 years or older, with no history of SARS-CoV-2, who were vaccinated between 20 December 2020 and 3 June 2021. A total of 10,861 vaccinated pregnant women were matched to 10,861 unvaccinated pregnant controls using demographic and clinical characteristics. Study outcomes included documented infection with SARS-CoV-2, symptomatic COVID-19, COVID-19-related hospitalization, severe illness and death. Estimated vaccine effectiveness from 7 through to 56 d after the second dose was 96% (95% confidence interval 89–100%) for any documented infection, 97% (91–100%) for infections with documented symptoms and 89% (43–100%) for COVID-19-related hospitalization. Only one event of severe illness was observed in the unvaccinated group and no deaths were observed in either group. In summary, the BNT162b2 mRNA vaccine was estimated to have high vaccine effectiveness in pregnant women, which is similar to the effectiveness estimated in the general population.
Similar content being viewed by others
Main
Hundreds of millions of individuals worldwide have been infected by the SARS-CoV-2 virus and millions have died from COVID-19 and related complications. Vaccines are currently the leading approach for combating the pandemic’s advance. Phase 3 clinical trials conducted to evaluate the safety and efficacy of mRNA COVID-19 vaccines did not include pregnant women, even though they are at risk for severe COVID-191 and potentially for adverse pregnancy outcomes2. Ongoing trials3 are limited to late pregnancy vaccination and surrogate efficacy outcomes (that is, immunogenicity). Given the current lack of evidence regarding the safety and efficacy of the vaccines for this population4,5, vaccination guidelines6,7,8 for pregnant women have been inconsistent, ranging from contraindicated to permitted to recommended in pregnancy.
As the number of vaccinated individuals increases worldwide, there is an opportunity to evaluate the real-world effectiveness and safety of the mRNA COVID-19 vaccines using observational data. Initial reports regarding vaccine safety indicate no obvious safety signals among pregnant women9. However, information regarding vaccine effectiveness among pregnant women is still limited10.
The immune system is known to undergo alterations during pregnancy. For example, there is evidence that levels of CD4+ and CD8+ lymphocytes decrease during pregnancy, as do the levels of some inflammatory cytokines11. Because mRNA-based vaccines are a new technology that has not been widely tested in pregnant women, it is plausible that the immune response triggered by these vaccines in pregnant women may be altered compared to the general population, increasing the need to evaluate vaccine effectiveness specifically for this subpopulation. In a previous report, confidence in vaccine effectiveness among pregnant women was mentioned as one of the strongest predictors of COVID-19 vaccine acceptance in this group12.
The objective of this study was to estimate real-world vaccine effectiveness in a large observational cohort of pregnant women, aged 16 years or older, with no previous SARS-CoV-2 infection and recruited between 20 December 2020 and 3 June 2021. Vaccinated women were exactly matched to unvaccinated controls on a set of demographic and clinical characteristics and followed for a median of 77 d. Vaccine effectiveness was estimated in several follow-up periods for documented infection with SARS-CoV-2, symptomatic COVID-19, COVID-19-related hospitalization, severe COVID-19 and COVID-19-related death.
Results
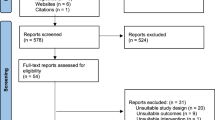
Of 38,836 women members of Clalit Health Services (CHS) vaccinated during pregnancy, 28,227 met the eligibility criteria (Methods); 10,861 of these vaccinated women were successfully matched to unvaccinated pregnant controls (Extended Data Fig. 1). The full population was similar to the eligible population (Supplementary Table 1). Matched individuals were also similar to the eligible population, albeit with a lower prevalence of some risk factors for severe COVID-19 (Supplementary Table 1).
The baseline characteristics of the matched individuals were very similar in the vaccinated and unvaccinated groups (Supplementary Table 2). The median age was 30 years, with 26%, 48% and 26% of pregnancies in the first, second and third trimesters, respectively. Of the matched individuals, 18% had at least 1 risk factor for severe COVID-19, the most common being obesity.
During a median follow-up of 77 d, 131 infections were documented in the vaccination group and 235 infections in the control group (Fig. 1). Event counts in each of the analysis periods (for individuals who were eligible to be included in the analysis for that period) are included in Supplementary Table 3. Cumulative incidence curves from baseline (first vaccine dose for the vaccinated group) are shown in Fig. 1 for the documented infection outcome and in Extended Data Figs. 2–4 for the additional outcomes. The curves in the vaccinated and unvaccinated groups are similar until day 14, when incidence in the vaccinated group begins to decline sharply.
Cumulative incidence curves of the documented infection outcome in pregnant women, CHS, 20 December 2020 through to 3 June 2021. The main line is the point estimate of the cumulative incidence and the shaded areas are the 95% CIs. The vertical lines at days 28 and 77 demarcate the period when an individual was considered ‘fully vaccinated’ in the manuscript (7 through to 56 d after receipt of the second dose). The curve represents the numbers at risk at each time point, along with the cumulative number of events. Each individual was included only once in each study group but individuals could move from the unvaccinated to the vaccinated group after receipt of the vaccine.
The estimated vaccine effectiveness for documented infections was 67% (95% confidence interval (CI) = 40–84%) in days 14–20 after the first dose, 71% (33–94%) in days 21–27 after the first dose and 96% (89–100%) in days 7–56 after the second dose (Table 1). The estimated vaccine effectiveness for symptomatic infection was 66% (95% CI = 32–86%) in days 14–20 after the first dose, 76% (30–100%) in days 21–27 after the first dose and 97% (91–100%) in days 7–56 after the second dose. Vaccine effectiveness for COVID-19-related hospitalization was 89% (43–100%) in days 7–56 after the second dose. Vaccine effectiveness could not be meaningfully estimated for the other outcomes and time periods due to the small number of events.
Discussion
In this study, we estimated that the BNT162b2 mRNA COVID-19 vaccine is as effective for pregnant women as previously reported for the general population during the same time period: 96% effectiveness against documented infection and 97% effectiveness against symptomatic infection 7–56 d after receipt of the second vaccine dose. The estimated vaccine effectiveness for COVID-19-related hospitalization was high but a paucity of cases prevented precise estimation. These results reflect the effectiveness mainly against the original SARS-CoV-2 reference strain and the B.1.1.7 (Alpha) variant, which were the dominant strains circulating in Israel during the study period.
Since the original phase 3 trials did not include pregnant women, data regarding the effectiveness of the new mRNA COVID-19 vaccines in this population is still scarce in the medical literature. One study confirmed the immunogenicity of these vaccines in pregnant women by confirming cellular and humoral immune responses against SARS-CoV-2 (ref. 13).
We previously estimated the vaccine effectiveness of the BNT162b2 mRNA COVID-19 vaccine for a general population using the data repositories of the same healthcare organization and covering the same variant distribution. After the second vaccine dose, vaccine effectiveness was estimated to be 92% (88–95%) for documented infections and 94% (87–98%) for symptomatic infections14. In an updated analysis of the same data over a longer period, the 95% CIs were narrower, with estimates of 93% (91–94%) for documented infection and 96% (94–97%) for symptomatic infection15. Our study suggests that the vaccine effectiveness estimate for pregnant women is not lower than that for the general population. This is consistent with the lower prevalence of comorbidities (some of which were associated with lower vaccine effectiveness15) in pregnant women compared with the general population. Our findings make it plausible that the vaccine effectiveness estimated in the general population for future variants may be used to infer the effectiveness in pregnant women for the same variants, particularly for mRNA-based vaccines.
Vaccination of pregnant women may also provide protection for their newborns. A recent study found binding and neutralizing antibodies in the cord blood of infants born to mothers who were vaccinated with mRNA vaccines and in the mothers’ breast milk13. Another study found that vaccination of breastfeeding women resulted in a rapid increase of anti-SARS-CoV-2-specific antibodies in their breast milk16. The magnitude and duration of this potential protection is still unclear.
The high vaccine effectiveness in pregnant women estimated in this study might also contribute to increased vaccination acceptance rates among this population. It was previously reported that high vaccine effectiveness is an important factor for the encouragement of pregnant women to receive COVID-19 vaccines. If vaccine effectiveness >90% was achieved, 52% of pregnant women reported that they would be willing to receive the vaccine. Furthermore, pregnant women indicated higher likelihood of vaccination with higher vaccine effectiveness12.
This study has several limitations. First, despite the careful matching between cohorts, there is the lingering possibility of residual confounding. This is particularly true because information regarding prenatal complications was not available. However, the very similar incidence of documented and symptomatic infections between the two study groups during the early period after the first vaccine dose suggests that residual confounding, if present, was minor. Second, owing to the low incidence of the more severe outcomes, this study could not provide precise vaccine effectiveness estimates for them. Third, the strict matching process required to achieve exchangeability between the study groups resulted in a relatively large fraction of the eligible population not being included in the study. Thus, the proportion of women with some chronic conditions was somewhat lower in the final study population. Vaccine effectiveness for women with chronic conditions may be somewhat lower than the average vaccine effectiveness estimated in this study, as previously reported for the general population15.
In conclusion, the results of this study indicate that the BNT162b2 mRNA COVID-19 vaccine is highly effective in pregnant women for the variants circulating in Israel at the time of the study, with vaccine effectiveness that was comparable to that estimated in the general population14. Further studies are needed to better characterize the dynamics of vaccine effectiveness throughout pregnancy, the relationship between vaccination timing and infant protection after birth and pregnancy- and non-pregnancy-related safety outcomes.
Methods
Ethics
This study was approved by the CHS community institutional review board.
Data source
CHS is an integrated healthcare payer-provider organization that serves 52% of the Israeli population. Medical insurance in Israel is mandatory for all residents, and covers a wide range of services, including prenatal care. The CHS population is fairly representative of the general Israeli population17. The present study was based on CHS data covering patients vaccinated from the start of the vaccination campaign in Israel on 20 December 2020 through to 3 June 2021. CHS data systems contain medical and claims data covering all facets of patient care, including primary care, specialist care, imaging, laboratory diagnostics and hospitalizations, with over 20 years of historical depth for most individuals. CHS community care includes dedicated systems for prenatal care, with specific data fields for ‘date of last menstrual period’ and ‘projected birth date’. Pregnancies are recorded in these dedicated systems from the moment a woman begins prenatal care, which is freely and universally available in Israel. These data are integrated daily with data collected centrally by the Israeli Ministry of Health regarding COVID-19 vaccines, SARS-CoV-2 tests and COVID-19-related hospitalizations, disease severity and death.
Study design and study population
We conducted an observational cohort study that emulates a target trial to estimate the effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnant women. We used a similar methodology to previous studies on vaccine effectiveness using the same database14. Eligibility criteria considered at the start of follow-up were pregnancy (as recorded in the CHS data systems), age of 16 years or older, continuous membership in CHS for 1 complete year, no previous positive SARS-CoV-2 PCR test, no previous SARS-CoV-2 vaccination, not residing in long-term care facilities, no home confinement due to medical reasons, not being a healthcare worker and no interaction with the healthcare system (physician appointment, laboratory test or hospitalization) in the previous 2 d (since this may signal a preexisting SARS-CoV-2 infection). Individuals with missing data (only relevant for the body mass index and living area variables) were excluded, as these are rare in the CHS data.
Each day during the study period, eligible women vaccinated on that day were individually matched to eligible women who had not yet been vaccinated and who were not previously matched as controls. Matching factors included age (in 3-year bins), trimester of pregnancy, geostatistical living area (corresponding to a small town or a single neighborhood within a large city or city/town of residence when the smaller geostatistical living area was not available), population sector (General Jewish, Arab or Ultraorthodox Jewish), count of influenza vaccinations in the last 5 years (in 2 bins) and existence of at least 1 Centers for Disease Control and Prevention risk factor for severe COVID-19 (ref. 18). Definitions for all variables used in the study are included in Supplementary Table 4.
The outcomes studied were: documented SARS-CoV-2 infection, defined as a positive SARS-CoV-2 PCR test; symptomatic SARS-CoV-2 infection (COVID-19), defined as an infection accompanied by the documentation of COVID-19 symptoms in dedicated fields in the outpatient health record (or an infection that warranted hospitalization); COVID-19-related hospitalization; severe COVID-19, as defined by the Israeli Ministry of Health using international criteria19; and COVID-19-related death. The outcome date was set to the date of the first positive test for the first two outcomes and the date of first occurrence for the latter outcomes.
Statistical analysis
After matching, we used the Kaplan–Meier estimator to construct cumulative incidence curves. We estimated vaccine effectiveness at different periods after vaccination: days 14–20 after the first dose; days 21–27 after the first dose; and days 7–56 after the second dose. In each period, we restricted the analysis to matched pairs where both members were not censored and had not developed the outcome before the beginning of that period. We then calculated the risk ratio (RR) and risk difference (RD) during that period, with vaccine effectiveness defined as 1 − RR. To estimate the analog of a per-protocol effect, we censored both members of the matched pair when the control received a vaccination but then allowed the control to be re-recruited as a vaccinated individual if a matched control was found. 95% CIs were estimated using the nonparametric percentile bootstrap method with 1,000 repetitions. Analysis was performed using R v.4.0.4.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Due to national and organizational data privacy regulations, individual-level data such as those used for this study cannot be shared openly.
Code availability
The modeling in this paper used R v.4.0.4 and the tidyverse v.1.3.0, survival v.3.2-7, survminer v.0.4.7 and boot v.1.3-27 R packages, all of which are freely available.
References
Zambrano, L. D. et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. MMWR Morb. Mortal. Wkly. Rep. 69, 1641–1647 (2020).
Wei, S. Q., Bilodeau-Bertrand, M., Liu, S. & Auger, N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ 193, E540–E548 (2021).
BioNTech SE. Study to Evaluate the Safety, Tolerability, and Immunogenicity of SARS CoV-2 RNA Vaccine Candidate (BNT162b2) Against COVID-19 in Healthy Pregnant Women 18 Years of Age and Older https://clinicaltrials.gov/ct2/show/NCT04754594 (2021).
Rubin, R. Pregnant people’s paradox—excluded from vaccine trials despite having a higher risk of COVID-19 complications. JAMA 325, 1027–1028 (2021).
Rasmussen, S. A., Kelley, C. F., Horton, J. P. & Jamieson, D. J. Coronavirus Disease 2019 (COVID-19) vaccines and pregnancy: what obstetricians need to know. Obstet. Gynecol. 137, 408–414 (2021).
Goodman, T. Update on WHO Interim Recommendations on COVID-19 Vaccination of Pregnant and Lactating Women https://cdn.who.int/media/docs/default-source/2021-dha-docs/update-on-who-interim-recommendations-on-c-19-vaccination-for-pregnant-and-lactating-women-70-.pdf (2021).
Centers for Disease Control and Prevention. COVID-19 Vaccines While Pregnant or Breastfeeding https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html (2021).
American College of Obstetricians and Gynecologists. COVID-19 Vaccination Considerations for Obstetric–Gynecologic Care https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care (2021).
Shimabukuro, T. T. et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N. Engl. J. Med. 384, 2273–2282 (2021).
Riley, L. E. & Jamieson, D. J. Inclusion of pregnant and lactating persons in COVID-19 vaccination efforts. Ann. Intern. Med. 174, 701–702 (2021).
Kourtis, A. P., Read, J. S. & Jamieson, D. J. Pregnancy and infection. N. Engl. J. Med. 370, 2211–2218 (2014).
Skjefte, M. et al. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur. J. Epidemiol. 36, 197–211 (2021).
Collier, A.-R. Y. et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA 325, 2370–2380 (2021).
Dagan, N. et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 384, 1412–1423 (2021).
Barda, N., Dagan, N. & Balicer, R. D. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. Reply. N. Engl. J. Med. 384, 1970 (2021).
Perl, S. H. et al. SARS-CoV-2-specific antibodies in breast milk after COVID-19 vaccination of breastfeeding women. JAMA 325, 2013–2014 (2021).
Cohen, R. & Rabin, H. Membership in the Health Funds https://www.btl.gov.il/Publications/survey/Documents/seker289/seker_289.pdf (2017).
Centers for Disease Control and Prevention. Certain Medical Conditions and Risk for Severe COVID-19 Illness https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html (2021).
National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines https://www.covid19treatmentguidelines.nih.gov/ (2021).
Acknowledgements
This study was supported by the Ivan and Francesca Berkowitz Family Living Laboratory Collaboration at Harvard Medical School and Clalit Research Institute. M.L. was supported by the Morris-Singer Foundation.
Author information
Authors and Affiliations
Contributions
S.H.D., B.Y.R. and R.D.B. contributed equally as senior authors to this study. N.D., N.B., T.B.S., C.K., S.H.D., B.Y.R. and R.D.B. conceived and designed the study. N.D., N.B. and M.M.A. participated in data extraction and analysis. N.D., N.B., I.S.K., M.A.H., M.L., S.H.D., B.Y.R. and R.D.B. wrote the manuscript. T.B.S. and S.H.D. provided clinical guidance. All authors critically reviewed the manuscript and decided to proceed with publication. B.Y.R. and R.D.B. supervised the study. R.D.B. vouches for the data and analysis.
Corresponding author
Ethics declarations
Competing interests
N.D., N.B., M.M.A. and R.D.B. report institutional grants to the Clalit Research Institute from Pfizer outside the submitted work and unrelated to COVID-19, with no direct or indirect personal benefits. M.L. reports grants from Pfizer, personal fees from Merck, Bristol Meyers Squibb, Sanofi Pasteur and Janssen, grants from the National Institutes of Health (NIH), the National Institute for Health Research, Centers for Disease Control and Prevention, Open Philanthropy Project, Wellcome Trust and Pfizer outside the submitted work; he has provided unpaid advice on COVID vaccines or vaccine studies to 1Day Sooner (nonprofit), Pfizer, AstraZeneca, Janssen and COVAXX (United Biosciences). M.A.H. reports grants from the NIH and Veterans Affairs, and personal fees from Cytel and ProPublica. S.H.D. reports consulting fees from Roche and UCB outside the submitted work. B.Y.R. reports grants from the NIH outside the submitted work. The other authors declare no competing interests.
Additional information
Peer review information Nature Medicine thanks Shabir Madhi, Aziz Sheikh and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Jennifer Sargent was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Population flow chart.
Size and percentage change of study population resulting from each inclusion and exclusion criteria. The figure focuses on the vaccinated population.
Extended Data Fig. 2 Cumulative incidence of SARS-CoV-2 symptomatic infection in vaccinated pregnant women and matched controls.
Cumulative incidence curves of the SARS-CoV-2 symptomatic infection outcome in pregnant women, Clalit Health Services, December 20, 2020 through June 3, 2021. The main line is the point estimate of the cumulative incidence and the shaded areas are 95% confidence intervals. The vertical lines at days 28 and 77 demarcate the period in which an individual is considered ‘fully vaccinated’ in the manuscript (7 through 56 days after receipt of the second dose). The table below the curve presents the number at risk at each time point, along with the cumulative number of events. Each individual was included only once in each study group, but individuals could move from the unvaccinated group to the vaccinated group after receipt of the vaccine.
Extended Data Fig. 3 Cumulative incidence of COVID-19 hospitalization in vaccinated pregnant women and matched controls.
Cumulative incidence curves of the COVID-19 hospitalization outcome in pregnant women, Clalit Health Services, December 20, 2020 through June 3, 2021. The main line is the point estimate of the cumulative incidence and the shaded areas are 95% confidence intervals. The vertical lines at days 28 and 77 demarcate the period in which an individual is considered ‘fully vaccinated’ in the manuscript (7 through 56 days after receipt of the second dose). The table below the curve presents the number at risk at each time point, along with the cumulative number of events. Each individual was included only once in each study group, but individuals could move from the unvaccinated group to the vaccinated group after receipt of the vaccine.
Extended Data Fig. 4 Cumulative incidence of severe COVID-19 in vaccinated pregnant women and matched controls.
Cumulative incidence curves of the severe COVID-19 outcome in pregnant women, Clalit Health Services, December 20, 2020 through June 3, 2021. The main line is the point estimate of the cumulative incidence and the shaded areas are 95% confidence intervals. The vertical lines at days 28 and 77 demarcate the period in which an individual is considered ‘fully vaccinated’ in the manuscript (7 through 56 days after receipt of the second dose). The table below the curve presents the number at risk at each time point, along with the cumulative number of events. Each individual was included only once in each study group, but individuals could move from the unvaccinated group to the vaccinated group after receipt of the vaccine.
Supplementary information
Supplementary Information
Supplementary Tables 1–4
Rights and permissions
About this article
Cite this article
Dagan, N., Barda, N., Biron-Shental, T. et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med 27, 1693–1695 (2021). https://doi.org/10.1038/s41591-021-01490-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-021-01490-8
This article is cited by
-
A narrative review of COVID-19 vaccination in pregnancy and breastfeeding
Journal of Perinatology (2024)
-
A qualitative study of pregnant women’s perceptions and decision-making regarding COVID-19 vaccination in Thailand
Scientific Reports (2024)
-
Comparative effectiveness of alternative intervals between first and second doses of the mRNA COVID-19 vaccines
Nature Communications (2024)
-
COVID-19 vaccination during pregnancy: a systematic review and meta-analysis
BMC Pregnancy and Childbirth (2023)
-
Third dose mRNA vaccination against SARS-CoV-2 reduces medical complaints seen in primary care: a matched cohort study
BMC Medicine (2023)