Abstract
Background
The liver has a solid inbuilt antioxidant defense system to regulate oxidative stress. However, exposure to an excessive level of ROS causes liver injury. This study examined the cytoprotective effect of neoxanthin, a xanthophyll antioxidant molecule isolated from Solanum trilobatum in stress-induced HepG2 cells.
Methods and results
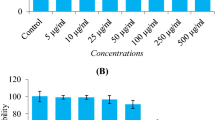
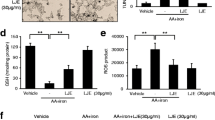
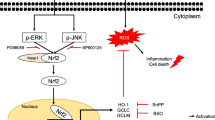
The cytotoxic effect of H2O2 and cytoprotective potential of β-carotene, lutein, and neoxanthin was analyzed by WST-1 assay. The intracellular ROS level and mitochondrial membrane potential (MMP) were measured using DCFH-DA (2′, 7′-dichlorofluorescin diacetate) and JC-10 MMP assay. The expression of anti-oxidant and apoptotic markers was measured by western blot analysis. Neoxanthin pretreatment exhibited better protection than β-carotene and lutein against cell death caused by H2O2. It significantly arrested H2O2-mediated elevation of intracellular ROS levels and protected MMP. The intracellular antioxidant enzymes HO-1 and SOD-2 were upregulated by neoxanthin pretreatment. Neoxanthin also activated the protein expression of redox-sensitive transactivation factors, Nrf2 and NF-kB. The cytoprotective effect of neoxanthin was associated with increased expression of the anti-apoptotic protein, Bcl-2 and decreased pro-apoptotic protein Bax.
Conclusions
For the first time, our results demonstrate that neoxanthin offers adequate protection against stress-mediated cytotoxicity in hepatocytes by activating the intracellular antioxidant defense system and blocking apoptosis.





Similar content being viewed by others
References
Prieto I, Monsalve M (2017) ROS homeostasis, a key determinant in liver ischemic-preconditioning. Redox Biol 12:1020–1025
Asrani SK, Devarbhavi H, Eaton J, Kamath PS (2019) Burden of liver diseases in the world. J Hepatol 70:151–171
Grewal US, Walia G, Bakshi R, Chopra S (2018) Hepatitis B and C viruses, their coinfection and correlations in chronic liver disease patients: a tertiary care hospital study. Int J Appl Basic Med Res 8:204
Redza-Dutordoir M, Averill-Bates DA (2016) Activation of apoptosis signalling pathways by reactive oxygen species. BBA-Mol Cell Res 1863:2977–2992
Luangmonkong T, Suriguga S, Mutsaers HA, Groothuis GM, Olinga P, Boersema M (2018) Targeting oxidative stress for the treatment of liver fibrosis. Rev Physiol Biochem Pharmacol 175:71–102
Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK (2018) Reactive oxygen species in metabolic and inflammatory signaling. Circ Res 122:877–902
Subedi RP, Vartak RR, Kale PG (2017) Management of stress exerted by hydrogen peroxide in Drosophila melanogaster using Abhrak bhasma. J Appl Pharm Sci 7:065–071
Singh M, Sharma H, Singh N (2007) Hydrogen peroxide induces apoptosis in HeLa cells through the mitochondrial pathway. Mitochondrion 7:367–373
Marinho HS, Real C, Cyrne L, Soares H, Antunes F (2014) Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol 2:535–562
Kohlgrüber S, Upadhye A, Dyballa-Rukes N, McNamara CA, Altschmied J (2017) Regulation of transcription factors by reactive oxygen species and nitric oxide in vascular physiology and pathology. Antioxid Redox Signal 26:679–699
Stephen NM, Gayathri R, Niranjana R, Prasad Y, Das AK, Baskaran V, Ganesan P (2017) Carotenoids: types, sources, and biosynthesis. Plant secondary metabolites, vol 2. Apple Academic Press, Palm Bay, pp 103–132
Ganesan P, Noda K, Manabe Y, Ohkubo T, Tanaka Y, Maoka T et al (2011) Siphonaxanthin, a marine carotenoid from green algae, effectively induces apoptosis in human leukemia (HL-60) cells. BBA-Gen Subj 1810:497–503
Sowmya Shree G, Prasad KY, Arpitha HS, Deepika UR, Kumar KN, Mondal P, Ganesan P (2017) β-carotene at physiologically attainable concentration induces apoptosis and down-regulates cell survival and antioxidant markers in human breast cancer (MCF-7) cells. Mol Cell Biochem 436:1–12
Kavalappa YP, Gopal SS, Ponesakki G (2021) Lutein inhibits breast cancer cell growth by suppressing antioxidant and cell survival signals and induces apoptosis. J Cell Physiol 236:1798–1809
Gopal SS, Maradgi T, Ponesakki G (2019) Anti-obese properties of carotenoids: an overview of underlying molecular mechanisms. Carotenoids: properties, processing and applications. Elsevier Academic Press, Cambridge
Shivarudrappa AH, Ponesakki G (2020) Lutein reverses hyperglycemia-mediated blockage of Nrf2 translocation by modulating the activation of intracellular protein kinases in retinal pigment epithelial (ARPE-19) cells. J Cell Commun Signal 14:207–221
Berni P, Chitchumroonchokchai C, Canniatti-Brazaca SG, De Moura FF, Failla ML (2015) Comparison of content and in vitro bioaccessibility of provitamin A carotenoids in home cooked and commercially processed orange fleshed sweet potato (Ipomea batatas Lam). Plant Foods Hum Nutr 70:1–8
Gopal SS, Eligar SM, Vallikannan B, Ponesakki G (2021) Inhibitory efficacy of lutein on adipogenesis is associated with blockage of early phase regulators of adipocyte differentiation. BBA-Mol Cell Biol Lipids 1866:158812
Kotake-Nara E, Asai A, Nagao A (2005) Neoxanthin and fucoxanthin induce apoptosis in PC-3 human prostate cancer cells. Cancer Lett 220:75–84
Kotake-Nara E, Sugawara T, Nagao A (2005) Antiproliferative effect of neoxanthin and fucoxanthin on cultured cells. Fish Sci 71:459–461
Kirtikar KR, Basu BS (1935) Solanacea. In: Blatler E, Cauis JC, Mhaskar KS (eds) Indian Medicinal Plants , vol 3, 2nd edn. Oriental Enterprises, Dehradun, pp 403–407
Chiu Y, Lo H, Huang H, Chao P, Hwang L, Huang P et al (2013) The antioxidant and cytoprotective activity of Ocimum gratissimum extracts against hydrogen peroxide-induced toxicity in human HepG2 cells. J Food Drug Anal 21:253–260
Ma Z, Li C, Qiao Y, Lu C, Li J, Song W et al (2016) Safflower yellow B suppresses HepG2 cell injury induced by oxidative stress through the AKT/Nrf2 pathway. Int J Mol Med 37:603–612
Kavalappa YP, Rudresh DU, Gopal SS, Shivarudrappa AH, Stephen NM, Rangiah K, Ponesakki G (2019) β-carotene isolated from the marine red alga, Gracillaria sp. potently attenuates the growth of hepatocellular carcinoma (HepG2) cells by modulating multiple molecular pathways. J Funct Foods 52:165–176
Böhm V, Lietz G, Olmedilla-Alonso B, Phelan D, Reboul E, Bánati D et al (2021) From carotenoid intake to carotenoid blood and tissue concentrations–implications for dietary intake recommendations. Nutr Rev 79:544–573
Niranjana R, Gayathri R, Mol SN, Sugawara T, Hirata T, Miyashita K, Ganesan P (2015) Carotenoids modulate the hallmarks of cancer cells. J Funct Foods 18:968–985
Cheng J, Liu D, Zhao J, Li X, Yan Y, Wu Z et al (2019) Lutein attenuates oxidative stress and inhibits lipid accumulation in free fatty acids-induced HepG2 cells by activating the AMPK pathway. J Funct Foods 60:103445
Liu Y, Zhang Y, Lin K, Zhang DX, Tian M, Guo HY et al (2014) Protective effect of piperine on electrophysiology abnormalities of left atrial myocytes induced by hydrogen peroxide in rabbits. Life Sci 94(2):99–105
Rao W, Zhang L, Su N, Wang K, Hui H, Wang L et al (2013) Blockade of SOCE protects HT22 cells from hydrogen peroxide-induced apoptosis. Biochem Biophy Res Commun 441:351–356
Xu X, Hang L, Huang B, Wei Y, Zheng S, Li W (2013) Efficacy of ethanol extract of Fructus lycii and its constituent’s lutein/zeaxanthin in protecting retinal pigment epithelium cells against oxidative stress: in vivo and in vitro models of age-related macular degeneration. J Opthamol 2013:862806
Li W, Zhang J, An W (2010) The conserved CXXC motif of hepatic stimulator substance is essential for its role in mitochondrial protection in H2O2-induced cell apoptosis. FEBS Lett 584:3929–3935
Qi G, Mi Y, Fan R, Li R, Wang Y, Li X et al (2017) Tea polyphenols ameliorate hydrogen peroxide and constant darkness-triggered oxidative stress via modulating the Keap1/Nrf2 transcriptional signaling pathway in HepG2 cells and mice liver. RSC Adv 7:32198
Chen C, Liu T, Chen C, Wong CH, Chen C, Lu F, Chen SC (2007) The efficacy of protective effects of tannic acid, gallic acid, ellagic acid, and propyl gallate against hydrogen peroxide-induced oxidative stress and DNA damages in IMR-90 cells. Mol Nutr Food Res 51:962–968
Park WH (2013) The effects of exogenous H2O2 on cell death, reactive oxygen species and glutathione levels in calf pulmonary artery and human umbilical vein endothelial cells. Int J Mol Med 31:471–476
Ramya S, Rajasekaran C, Sivaperumal R, Krishnan A, Jayakumararaj R (2008) Ethnomedicinal perspectives of botanicals used by Malayali Tribes in Vattal Hills of Dharmapuri (TN), India. Ethnobot Leafl 12:1054–1060
Emmanuel S, Ignacimuthu S, Perumalsamy R, Amalraj T (2006) Anti-inflammatory activity of Solanum trilobatum. Fitoterapia 77:611–612
Ranjith MS, Ranjitsingh AJA, Shankar SG, Vijayalaksmi GS, Deepa K, Babu K, Sidhu HS (2010) Solanum trilobatum in the management of atopy: through inhibition of mast cell degranulation and moderation of release of interleukins. Pharmacogn Res 2:10–14
Ahmed Z, Syed K, Ahmed Sidhra SZ, Ponmurugan P, Kumar BS (2016) Ameliorative potential of Solanum trilobatum leaf extract and fractions on lipid profile and oxidative stress in experimental diabetes. Pak J Pharm Sci 29:1578
Shahjahan M, Vani G, Shyamaladevi CS (2005) Effect of Solanum trilobatum on the antioxidant status during diethyl nitrosamine induced and phenobarbital promoted hepatocarcinogenesis in rat. Chem Biol Interact 156:113–123
Ganesan K, Sukalingam K, Xu B (2017) Solanum trilobatum L. ameliorate thioacetamide-induced oxidative stress and hepatic damage in albino rats. Antioxidants 6:68
Vijaimohan K, Devi CS, Mallika J (2010) Chemoprotective effect of sobatum against lithium-induced oxidative damage in rats. J Young Pharm 2:68–73
Sugawara T, Kushiro M, Zhang H, Nara E, Ono H, Nagao A (2001) Lysophosphatidylcholine enhances carotenoid uptake from mixed micelles by Caco-2 human intestinal cells. J Nutr 131:2921–2927
Asai A, Yonekura L, Nagao A (2008) Low bioavailability of dietary epoxyxanthophylls in humans. Br J Nutr 100:273–277
Biehler E, Hoffmann L, Krause E, Bohn T (2011) Divalent minerals decrease micellarization and uptake of carotenoids and digestion products into Caco-2 cells. J Nutr 141:1769–1776
Kotake-Nara E, Kushiro M, Zhang H, Sugawara T, Miyashita K, Nagao A (2001) Carotenoids affect proliferation of human prostate cancer cells. J Nutr 131:3303–3306
D’Andrea T, Hellmold H, Jonsson C, Zhivotovsky B, Hofer T, Wärngård L, Cotgreave I (2004) The transcriptosomal response of human A549 lung cells to a hydrogen peroxide-generating system: relationship to DNA damage, cell cycle arrest, and caspase activation. Free Radic Biol Med 36:881–896
Park J, Lee J, Choi C (2011) Mitochondrial network determines intracellular ROS dynamics and sensitivity to oxidative stress through switching inter-mitochondrial messengers. PloS one 6:e23211
Heo SJ, Ko SC, Kang SM, Kang HS, Kim JP, Kim SH et al (2008) Cytoprotective effect of fucoxanthin isolated from brown algae Sargassum siliquastrum against H2O2-induced cell damage. Eur Food Res Technol 228:145–151
Kim Y, Seo JH, Kim H (2011) β-Carotene and lutein inhibit hydrogen peroxide-induced activation of NF-κB and IL-8 expression in gastric epithelial AGS cells. J Nutr Sci Vitaminol 57:216–223
Li Z, Dong X, Liu H, Chen X, Shi H, Fan Y et al (2013) Astaxanthin protects ARPE-19 cells from oxidative stress via upregulation of Nrf2-regulated phase II enzymes through activation of PI3K/Akt. Mol Vis 19:1656
Wang X, Cui YJ, Qi J, Zhu MM, Zhang TL, Cheng M, Liu SM, Wang GC (2018) Fucoxanthin exerts cytoprotective effects against hydrogen peroxide-induced oxidative damage in L02 cells. Bio Med Res Int 2018:1–11
Sun X, Jia H, Xu Q, Zhao C, Xu C (2019) Lycopene alleviates H2O2-induced oxidative stress, inflammation and apoptosis in bovine mammary epithelial cells via the NFE2L2 signaling pathway. Food Funct 10:6276–6285
Lin J, Xia J, Zhao HS, Hou R, Talukder M, Yu L et al (2018) Lycopene triggers Nrf2–AMPK cross talk to alleviate atrazine-induced nephrotoxicity in mice. J Agric Food Chem 66:12385–12394
Feng C, Luo T, Zhang S, Liu K, Zhang Y, Luo Y, Ge P (2016) Lycopene protects human SH-SY5Y neuroblastoma cells against hydrogen peroxide-induced death via inhibition of oxidative stress and mitochondria-associated apoptotic pathways. Mol Med Rep 13:4205–4214
Levine B, Sinha SC, Kroemer G (2008) Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy 4:600–606
Fan Y, Dutta J, Gupta N, Fan G, Gélinas C (2008) Regulation of programmed cell death by NF-κB and its role in tumorigenesis and therapy. Programmed cell death in cancer progression and therapy. Springer, Dordrecht, pp 223–250
Bohn T (2019) Carotenoids and markers of oxidative stress in human observational studies and intervention trials: Implications for chronic diseases. Antioxidants 8:179
Acknowledgements
The author, TM, acknowledges the Department of Biotechnology (DBT), New Delhi, India, for granting a research fellowship. The authors thank the Director, CSIR-CFTRI, for the constant support to carry out this work.
Author information
Authors and Affiliations
Contributions
GP, DUR, NMS and TM designed the study; DUR, TM and NMS performed the experiments; GP, DUR, TM and NMS wrote the manuscript; AN and KNR contributed to data analysis and editing of the manuscript, and all the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Udayawara Rudresh, D., Maradagi, T., Stephen, N.M. et al. Neoxanthin prevents H2O2-induced cytotoxicity in HepG2 cells by activating endogenous antioxidant signals and suppressing apoptosis signals. Mol Biol Rep 48, 6923–6934 (2021). https://doi.org/10.1007/s11033-021-06695-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06695-1