Abstract
Recently, Curcuma rhizome-related foods with claimed health benefits have been used worldwide; however, correct identification and quality assessment have not been conducted. Due to the wide distribution and morphological similarities of Curcuma species, the classification of some species is debated and nomenclature is inconsistent among countries. In this study, to elucidate specific molecular markers of medicinally used Curcuma species in Asia, and to solve the confusion on the reported botanical origin of crude drugs, molecular analysis based on the intron length polymorphism (ILP) in genes encoding diketide-CoA synthase and curcumin synthase and the trnK intron sequences was performed using 59 plant specimens and 42 crude drug samples from 13 Curcuma species, obtained from Asian countries. The ILP patterns of the respective species from both plant specimens and crude drug samples revealed high consistency in C. aromatica, C. zedoaria, C. phaeocaulis, C. aeruginosa, C. wenyujin, and C. zanthorrhiza, but showed intraspecies polymorphism in C. longa, C. kwangsiensis, C. amada, C. mangga and C. comosa. The C. longa specimens and samples were separated into three subgroups which were highly consistent with their geographical origins. Based on the ILP markers and the trnK intron sequences, the botanical origins of “Khamin oi” from Thailand were correctly determined to be C. longa or a hybrid between C. longa and other species, and “Wan narn kum” from Thailand and “Kasturi manjal” from India were correctly determined to be C. zanthorrhiza.
Similar content being viewed by others
Introduction
Genus Curcuma (Zingiberaceae) comprises approximately 120 species that grow widely in subtropical and tropical Asia. The most widely distributed and economically valuable species C. longa L. is cultivated around the world. The dried rhizome of C. longa—called turmeric in English, “Ukon” in Japanese, “Jianghuang” in Chinese, “Haldi” in Hindi, “Khamin chan” in Thai and “Kunyir” in Javanese—has been used as a traditional crude drug, spice, dye, cosmetic as well as a health food in Asian countries. A number of other Curcuma species are cultivated and used in Asia: C. phaeocaulis Valeton, C. kwangsiensis S. G. Lee et C. F. Liang and C. wenyujin Y. H. Chen et C. Ling cultivated in China as the traditional Chinese crude drug “Ezhu”; C. zedoaria (Christm.) Roscoe and C. aromatica Salisb. cultivated in Japan as the crude drug “Gajyutsu” and the health food “Haru-ukon,” respectively; C. zedoaria, C. aromatica, C. zanthorrhiza Roxb., C. aeruginosa Roxb., C. caesia Roxb. and C. amada Roxb. in India as Ayurveda crude drugs, folk medicine or a source of starch; C. zedoaria, C. aromatica, C. comosa Roxb., C. aeruginosa and C. mangga Valeton et Zijp in Thailand as crude drugs or foods; C. zanthorrhiza, C. zedoaria, C. aeruginosa and C. heyneana Valeton et Zijp in Indonesia as crude drugs or a source of starch [1,2,3,4]. The medicinal properties of C. longa are mainly attributed to its abundant content of curcuminoids which have been reported to possess anti-inflammatory, antioxidant and anticancer activities [5,6,7]. However, other Curcuma drugs that contain no or few curcuminoids but characteristic essential oils also have pharmacological effects. For example, C. phaeocaulis rhizome showed anti-inflammatory activity [8] and cyclooxygenase-2 inhibitory activity in vitro, with furanodienone and curcumenol identified as the active constituents [9].
Recently, with the increasing popularity of foods with health claims and so-called “health food,” including those derived from Curcuma rhizomes in Japan and other countries, the Curcuma rhizomes mentioned above are frequently used worldwide; however, correct identification and quality assessment have not been performed. Due to the wide distribution and morphological similarities of Curcuma species, the classification of some species is debated and nomenclature is inconsistent among countries, especially for C. aromatica and C. zedoaria. This situation leads to confusion in the use of Curcuma crude drugs. Multiple DNA regions have been tested for their capability in discrimination of Curcuma species, including matK, rbcL, rpoC1, rpoB, rps36-rps8, ndhJ, trnL-F, trnH-psbA, accD and trnS-trnfM of chloroplast DNA [10,11,12,13] as well as internal transcribed spacer (ITS) regions of nuclear DNA [10, 13]. However, these chloroplast DNA regions revealed limited resolution due to the high conservation in their sequences [10,11,12,13]. The ITS sequences showed high polymorphism even within a single individual, thus a cloning method is required for further analyses [10, 13]. We performed molecular analysis on the trnK intron region of chloroplast DNA to discriminate six Curcuma species from China and Japan, and detected five main sequence types [14, 15]. Moreover, by using a new marker based on the intron length polymorphism (ILP) of genes encoding diketide-CoA synthase (DCS) and curcumin synthase (CURS), the two important enzymes involved in the biosynthesis of curcuminoids, there were distinguishable ILP patterns of C. longa, C. phaeocaulis, C. zedoaria, C. kwangsiensis, C. aromatica and C. wenyujin as well as intraspecies variation of C. longa and C. kwangsiensis [16]. The ILP markers, including PCR amplicons of two intron regions in the two DCS genes and one intron in the three CURS genes (Fig. S1), showed potential for discrimination of Curcuma plants and related products. However, the tested specimens and crude drug samples were mostly limited to those from China and Japan. Further study with a large sample size including Curcuma species and Curcuma-related crude drugs from Southeast and South Asia, such as Thailand, Indonesia and India is needed. The present study aims to elucidate specific ILP patterns of medicinally used Curcuma species in Asia, to locate the original habitats of some Curcuma species cultivated in Japan, and to resolve the confusion caused by inconsistent scientific names among countries, especially those reported as the botanical origin of crude drugs. To do this, molecular analysis based on the ILP markers of DCS and CURS genes and the trnK intron sequences was performed using a number of Curcuma specimens and crude drug samples obtained from Japan, China, Thailand, Indonesia, India, and other Asian countries.
Materials
Fifty-nine plant specimens of 11 Curcuma species including C. longa, C. aromatica, C. phaeocaulis, C. aeruginosa, C. zedoaria, C. zanthorrhiza, C. wenyujin, C. kwangsiensis, C. amada, C. petiolata Roxb. and C. sichuanensis X. X. Chen, and uncertain species such as C. mangga were mainly collected from several medicinal plant gardens in Japan (Table 1). Most of them were introduced from China, Thailand, Indonesia, India, Malaysia and Nepal. Forty-two crude drug samples were widely collected from various markets in Thailand, India, Indonesia, China, Japan, Myanmar, Nepal and Sri Lanka (Table 2). The vouchers were deposited in the Museum of Materia Medica, Institute of Natural Medicine, University of Toyama (TMPW). Botanical origins of crude drug samples (Table 2) were deduced from their local names by referring to the literature [3, 4, 17,18,19].
Methods
Morphology of plant specimens
With regard to plant specimens obtained from medicinal plant gardens, their morphological features including the internal color of rhizomes, color of the leaf sheath, presence or absence of a purple band on and around the leaf midvein, the presence or absence of hairs on upper and lower sides of leaves, the position of inflorescence and the color of bracts in terminal and lower parts of the inflorescence were compared with botanic literatures [3, 18,19,20,21] for morphological identification. Those already preserved in the TMPW museum were identified by Dr. Indira Balachandran, Center for Medicinal Plants Research, Arya Vaidya Sala, India, and Dr. Katsuko Komatsu, Institute of Natural Medicine, University of Toyama, Japan.
Isolation of total DNA
Total DNA was extracted from 40 to 50 mg of dried leaves of plant specimens or 80–100 mg of dried rhizomes of crude drug samples using a DNeasy Plant Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions with some modifications [22]. A 1-μL aliquot of extraction solution of each sample was examined using 1.0% agarose gel electrophoresis stained with UltraPower DNA Safedye (Gellex International, Japan) to check the condition of total DNA.
PCR amplification and sequence analysis of trnK intron regions
The trnK intron regions of plant specimens and crude drug samples were amplified via PCR using two pairs of primers [14, 15]: trnK3914F (5′-TGG GTT GCT AAC TCA ATG G-3′) and CT911R (5′-TAT AGA AAG TGT TGT TGC CG-3′) for upstream intron regions; and CT2240F (5′-TTG CAA AGA TTA AGT TCG GG-3′) and trnK2R (5′-AAC TAG TCG GAT GGA GTA G-3′) for the downstream intron regions (Fig. S2). Of PCR solution, 25 μL contained 10–100 ng of total DNA as a template, 1 × Buffer for KOD-Plus, 0.2 mM dNTPs, 1.0 mM MgSO4, 0.4 μM of each primer and 0.5 U of KOD-Plus polymerase (Toyobo, Japan). The PCR amplification was carried out with a Takara PCR Thermal Cycler TP600/650 (Takara, Japan). The cycling condition was a hot start at 94 °C for 4 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 50 °C for 30 s, extension at 68 °C for 60 s and a final extension at 68 °C for 7 min. For samples for which this PCR amplification failed, the KOD-FX Neo DNA polymerase system (Toyobo) was then used, in which 25 μL of solution consisted of 12.5 μL of 2 × Buffer for KOD-FX Neo, 0.2 mM dNTPs, 0.4 μM of each primer and 0.5 U of KOD-FX Neo polymerase. The PCR cycling condition was the same as that of the KOD-Plus system. A 1-μL PCR product of each sample was examined using 1.0% agarose gel electrophoresis. Then, the PCR products were purified using Wizard SV Gel and PCR Clean-Up System (Promega, USA).
The sequencing reaction was performed with 50 ng of each purified PCR product as a template and each of the primers CT23F (5′-AGT ACT CGG CTT TTA AGT GC-3′) or CT828R (5′-TGA AGC AGA GGT AGA AGG AAC-3′) for the upstream intron region and each of CT2240F or CT2675R (5′-TTT TCC TTG TTA TAA TAG GT-3′) for the downstream intron region [15, 16]. The 10 μL sequencing reaction mixture contained 1.8 μL of BigDye Sequencing Buffer (ThermoFisher, USA), 0.5 μL of BigDye Terminator v3.1 (ThermoFisher) and 0.35 μM primer. The cycling condition used for the sequencing reaction was a hot start at 96 °C for 1 min, followed by 26 cycles of denaturation at 96 ℃ for 10 s, annealing at 50 °C for 5 s and extension at 60 °C for 4 min. The sequencing reaction products were purified using BigDye XTerminatorTM Purification Kit (ThermoFisher), then sequences of the respective purified products were determined by an ABI Prism 3100-Avant Genetic Analyzer (ThermoFisher). Sequencing data were collected with 3100-Avant Data Collection Software (v5.3, ThermoFisher) and sequences were assembled with Sequencing Analysis Software (v5.3, ThermoFisher). Consensus sequences were aligned and compared using Multalin software (http://multalin.toulouse.inra.fr/multalin/) or BioEdit (ver. 4.0.6.2). The determined trnK intron sequences were registered in the International Sequence Database (INSD: DDBJ/EMBL/GenBank) with the accession numbers shown in Table 4.
PCR amplification and size determination of the amplicons of intron regions of DCS1, DCS2 and CURS1–CURS3
Two intron regions I and II in DCS1 and DCS2 and one intron region in CURS1–CURS3 were amplified separately via PCR using each of the three pairs of primers [16]: DCSI-F (5′-GAC TWC TAY TTC CGS GTC AC-3′) and DCSI-R (5′-GAG CCA GCA ARC TMG GAT TC-3′); DCSII-F (5′-CCA CAT CGA GAG CCT CTT CG-3′) and DCSII-R (5′-CTG GCT YTT SAG GTG GAA GGT C-3′); and CURS-F (5′-GAC TWC TAY TTC CGS GTC AC-3′) and CURS-R (5′-CTT SGG CCK CTS CTT CAG GAT C-3′). Primers DCSI-F, DCSII-R and CURS-R were labeled with fluorescent dyes 6-FAM, HEX and CY-3, respectively, which enabled the respective amplicons to be detected and discriminated. A KOD-Plus or KOD-FX Neo DNA Polymerase Kit was used for PCR amplification and the composition of the common ingredients in PCR solution was the same as that described in the section “PCR amplification and sequence analysis of trnK intron regions”. The cycling condition was a hot start at 98 °C for 4 min, followed by 35 cycles of denaturation at 98 °C for 10 s, annealing at 58 °C for 30 s, extension at 68 °C for 60 s and a final extension at 68 °C for 7 min. For each sample, 1 μL of PCR product was examined using 1.0% agarose gel electrophoresis. Successfully amplified fragments were diluted with dH2O in ratio range of 10–50. Then, 0.5 μL of diluted PCR product of each intron region was mixed with 9.0 μL of Hi-Di Formamide (ThermoFisher) and 0.5 μL of GeneScan 600 LIZ Size Standard (ThermoFisher). The size of the amplified fragments was determined by an ABI Prism 3100-Avant Genetic Analyzer (ThermoFisher) with GeneMapper software (ver. 3.5, ThermoFisher). Determining the fragment length in ILP analysis followed two steps: compared with size standard markers that included 36 single-stranded labeled fragments ranging within 20–600 bp; fragment length was calculated automatically and the length value was manually adjusted within ± 1 bp by rounding and comparing with reference samples of the respective species.
Dendrogram analysis
The fragment pattern in the three intron regions of each sample was converted to a binary character string by scoring each fragment as 1 (presence) or 0 (absent), and then a proportional distance matrix was generated using the PAST software (ver. 4.0). Cluster analysis was conducted and a dendrogram was constructed based on the matrix by neighbor-joining (NJ) method [23] using the MEGA X software (ver. 10.1.6) [24].
Results and discussion
Morphological identification of plant specimens
Fifty-two plant specimens were morphologically identified according to taxonomic literature [3, 18,19,20,21]. The informative morphological features for discrimination of Curcuma species including information on rhizomes, leaves and inflorescences were observed and summarized (Table 3).
The C. longa specimens had green-colored and glabrous leaves, and a bulbed rhizome with several finger-shaped rhizomes attached whose cut surface was bright yellow to orange. Two uncertain specimens 94009 and 97021 which had the above characteristics were identified as C. longa. The inside color of C. longa rhizome varied depending on the producing areas; for instance, it was bright yellow in China and Japan, orange in Thailand and reddish orange in Indonesia. The C. aromatica from Japan and China was discriminated from C. longa by the features of leaves with a pubescent lower surface, light-yellow rhizomes as well as separately arising inflorescences. Uncertain specimen 94006 had similar morphology to Japanese C. aromatica. C. phaeocaulis, C. aeruginosa, C. zedoaria and C. zanthorrhiza had a purple band along the midrib on the upper leaf surface; however, similarities and variabilities in this feature made it difficult to identify these species, particularly for C. phaeocaulis and C. aeruginosa which also had similar rust-colored leaf sheaths. The Flora of China mentions that C. phaeocaulis, C. aeruginosa, and C. zedoaria have long been misidentified in China [20]. Based on comparative and precise observations, the morphological differences between C. phaeocaulis and C. aeruginosa were summarized: C. phaeocaulis had a wide purple band on the upper leaf surface (Fig. 1A) and short hairs on the lower surface, lateral inflorescence and inside color of rhizomes was greenish-blue and yellow (Fig. 2A); whereas C. aeruginosa had a narrow purple band on the upper leaf surface (Fig. 1B) and a glabrous lower surface, central or lateral inflorescence and the rhizome interior was yellowish-green and yellow (Fig. 2B). According to these morphological characters, uncertain specimen 94010 was identified as C. phaeocaulis, with rust-colored leaf sheaths, a wide purple band on upper leaf surfaces and blue rhizome interior. Three specimens of C. zedoaria from Japan were characterized by green leaf sheaths, a purple band along the midvein from the middle to the tip of leaves (Fig. 1C) and rhizomes with pale or purplish-blue interior (Fig. 2C). The C. zanthorrhiza specimens were similar to C. zedoaria cultivated in Japan but were discriminated by yellowish-orange to orange rhizomes (Fig. 2D) and an extremely narrow purple band along the leaf midrib (Fig. 1D). The C. kwangsiensis specimens were characterized by dense hairs on both leaf surfaces and yellowish white rhizomes, although there were some variations such as in leaf sheath color, presence or absence of a purple band on the leaf blade and inflorescence position as previously reported [25]. Two Curcuma specimens, one labeled C. amada, originally obtained from India and another labeled C. mangga from Thailand had mango-smelling rhizomes with yellow and pale yellow inside color, green leaf sheaths and green leaves; however, other morphological features were unavailable. The C. petiolata specimen had oblong to ovate leaves with long petioles and pale-yellow rhizomes. Leaves of specimen 94008 had creamy white margins similar to some varieties of C. petiolata. The C. sichuanensis specimen had similar features to C. longa except for a light-yellow rhizome interior.
trnK intron sequences
The trnK intron sequences of all plant specimens and crude drug samples were successfully determined, except for plant specimens T004 and I-0007. In accord with our previous reports [14,15,16, 25], C. longa showed Ltk(11T) and Ltk(10T) types of sequences, and both C. kwangsiensis and C. wenyujin had a K(gl)Wtk type of sequence. In addition, C. aromatica (Jp), C. zedoaria (Jp) and C. phaeocaulis showed Atk, K(pl)Ztk and Ptk types of sequences, respectively (Table 4). In addition to these six types of recorded sequences, five new types were detected. Three new types showed high similarities to the type Ltk(11T) sequence, and were named Ltk(11T)in-1, Ltk(11T)in-2 and Ltk(11T)139A. The Ltk(11T)in-1 and -2 types had a 5-bp “CATAA” insertion either in front of or behind the sequence unit “TACAA” at the alignment positions 2433–2442; Ltk(11T)139A type had an adenine instead of guanine at position 139. The Ltk(11T)in-1 and -2 types were detected in two plant specimens of C. longa and two crude drug samples from Thailand. The Ltk(11T)139A type was detected in crude drug sample D8685 from Sri Lanka, which has been generally considered as C. longa [3]. The other two new types showed high similarities to the K(pl)Ztk type sequence but differed by the number of poly-adenine and poly-thymine observed from positions 205 and 502, respectively. The K(pl)Ztk type sequence detected in C. zedoaria from Japan had 6 adenines and 14 thymines at these two sites [renamed K(pl)Ztk(6A14T)], whereas new types had 7 adenines and 15 or 13 thymines [named K(pl)Ztk(7A15T) or K(pl)Ztk(7A13T), respectively]. The K(pl)Ztk(7A15T) sequence type was detected in five crude drug samples from Thailand. The K(pl)Ztk(7A13T) sequence type was detected in crude drug samples D21642, D24876 and D30519 from Thailand and crude drug sample D22836 from India.
There were four species for which the trnK intron sequences were analyzed for the first time: C. petiolata and C. sichuanensis had a Ltk(11T) sequence that was the same as for C. longa; C. zanthorrhiza had a K(pl)Ztk(6A14T) sequence that was the same as for C. zedoaria (Jp); and C. aeruginosa, C. amada and C. mangga had a Ptk sequence that was the same as for C. phaeocaulis. These species pairs as well as another pair, C. wenyujin and C. kwangsiensis, could not be discriminated using the trnK intron sequences. The trnK sequence types of crude drug samples are summarized in Table 2.
One C. kwangsiensis specimen (Q69) had a Ltk(10T)2207T type of sequence with a thymine instead of cytosine at position 2207 in Ltk(10T), but not a K(gl)Wtk type. As reported in our previous paper [25], C. kwangsiensis is probably of hybrid origin and several species including C. wenyujin, C. phaeocaulis and C. longa might be involved in its formation. The trnK intron sequence of Q69 suggested that this specimen might have a maternal line from C. longa because of the maternal heritance of the chloroplast DNA. The same phenomenon was detected in the crude drug sample “Kamin oi” from Thailand, which has been generally considered as the rhizome of C. zedoaria; samples D21642 and D24876 showed K(pl)Ztk(7A13T) type, and sample D24864 showed Ltk(11T) type, although these three samples had the same morphological features.
ILP analysis of DCS introns I and II and CURS intron regions
The three intron regions of 59 plant specimens and 41 crude drug samples were amplified successfully. The length of the amplified DNA fragments ranged from 213 to 276 bp in DCS intron I region, 274 to 308 bp in DCS intron II region and 194 to 256 bp in CURS intron region. The ILP patterns based on both number and length of the amplified fragments in the three intron regions are shown in Fig. 3. The ILP patterns revealed high intraspecies consistency in C. aromatica from Japan and China; C. zedoaria from Japan, India and Indonesia; and C. phaeocaulis, C. aeruginosa, C. wenyujin and C. zanthorrhiza; but showed intraspecies polymorphism in C. longa, C. kwangsiensis, C. amada, C. mangga and C. comosa.
ILP patterns of all plant specimens and crude drug samples. Voucher no. preceded by “D” indicates a crude drug sample. *The scientific name of crude drug samples was deduced from the local name, shown in parentheses. **[16], samples whose trnK intron sequences and ILP patterns were previously reported in reference [16]; [14, 15, 25], samples whose trnK intron sequences were previously reported in references [14, 15, 25]
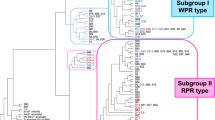
Based on similarities of the ILP patterns of all samples, an unrooted tree was constructed using the NJ method (Fig. 4). In the NJ tree, C. petiolata and C. comosa formed a clade, separated from the large clade comprising the rest of the species. The large clade was further divided into two subclades, in which all the plant specimens and crude drug samples of C. longa formed one subclade, and another subclade comprising the other species was further divided into two branches: one composed of C. aromatica, C. zedoaria, C. aeruginosa, C. phaeocaulis, C. wenyujin, and C. kwangsiensis; and the other composed of C. zanthorrhiza, C. amada and C. mangga. The similarity of ILP patterns in the specimens and samples of the respective species led to clear clustering in the NJ tree, with 11 main groups corresponding to the respective species: group L (C. longa), group JA (Japanese population of C. aromatica), group Ze (C. zedoaria), group Ae (C. aeruginosa), group P (C. phaeocaulis), group W (C. wenyujin), group K (C. kwangsiensis), group Za (C. zanthorrhiza), group A/M (C. amada or C. mangga), group Pe (C. petiolata) and group C (C. comosa).
Group L (C. longa) included all the plant specimens or crude drug samples of C. longa that were introduced from or produced in Japan, China, Thailand, Indonesia, India, Sri Lanka and Nepal, as well as plant specimen Q50 of C. sichuanensis from China and crude drug samples D24864, D21642 and D24876 from Thailand (Fig. 3). This group was further divided into three subgroups and this grouping was highly consistent with the geographical origins of the included samples. Therefore, they were tentatively assigned as China–Japan (L1), Thailand (L2) and India–Indonesia (L3) subgroups. In subgroup L1, six plant specimens of C. longa from Japan, China, and Nepal showed identical ILP patterns as well as trnK intron sequences, and plant specimen Q50 of C. sichuanensis also showed the same ILP pattern and the same Ltk(11T) type of trnK intron sequence as C. longa. According to the Flora of China [20], C. sichuanensis has strong morphological similarities to C. longa, only differing from C. longa by light inside color of rhizomes and coma bracts as well as a yellow band in the labellum. Xiao et al. suggested that C. sichuanensis should be treated as C. longa cv. sichuanensis or C. longa complex based on their field investigation in Sichuan, China [17]; the morphological and molecular evidence obtained in our study supports this proposal. Crude drug sample D24864 collected from Thailand was included in this subgroup. Although this sample was deduced to be C. zedoaria from its local name “Khamin oi,” the ILP pattern and Ltk(11T) type of trnK intron sequence of this sample suggested its botanic source might be a strain closely related to C. longa. In subgroup L2, three plant specimens and two crude drug samples of C. longa from Thailand showed identical ILP patterns (Fig. 3). Two crude drug samples D21642 and D24876 with the local name “Khamin oi” had the same ILP pattern, which somewhat differed from that of C. longa from Thailand, and their K(pl)Ztk(7A13T) type of trnK intron sequence differed from both that of C. longa and C. zedoaria. The above results suggest that these two “Khamin oi” samples might be of hybrid origin in which C. longa and species with a K(pl)Ztk(7A13T) type of trnK intron sequence were involved in hybridization. Further study is needed to clarify the botanical origin of “Khamin oi” and to investigate the variability of ILP pattern in hybrid plants. The subgroup L3 included seven plant specimens or crude drug samples from India, three plant specimens or crude drug samples from Indonesia and one crude drug sample from Sri Lanka. Except for six samples from India, the samples showed an identical ILP pattern. Five ILP patterns were detected in the seven Indian specimens and samples, indicating considerable genetic polymorphism of C. longa in India. In fact, C. longa is widely cultivated in India and a number of cultivars have been developed to facilitate cultivation in various locations [3, 4]. In this study, four crude drug samples derived from four cultivars showed different ILP patterns.
The group JA included ten plant specimens of C. aromatica, including six specimens cultivated in Japan, three introduced from China and one uncertain specimen from Thailand. In Japan, C. aromatica has long been widely cultivated; however, the original source remains unclear. Japanese C. aromatica showed the same ILP pattern and trnK intron sequences as the three C. aromatica specimens introduced from China and one specimen introduced from Thailand (Fig. 3), as well as C. chuanhuangjiang from China [16]. Unfortunately, the type specimen of C. aromatica was unavailable and it has been pointed out that the name of C. aromatica is applied to several taxa in Asia that possess similar morphological features such as pale brown and aromatic rhizomes, entirely green leaves with a glabrous upper surface and pubescent lower surface, and lateral inflorescences [3, 26, 27]. In this study, the crude drug samples from India (D20477, D20483 and D22836) and Thailand (D24867), deduced to be C. aromatica, genetically differed from the Japanese and Chinese C. aromatica (Fig. 3) and so partly reflected such a situation.
The group Ze included four plant specimens of C. zedoaria (three cultivated in Japan and one introduced from India) as well as four crude drug samples produced in Japan or Indonesia. These samples showed an identical ILP pattern and the same K(pl)Ztk(6A14T) type of trnK intron sequence, except one crude drug sample (D24986, generally considered to be C. aeruginosa according to its local name “Temu ireng”) which showed a Ptk type of trnK intron sequence (Fig. 3). Taxonomically, C. zedoaria is quite similar to C. aromatica, due to historical nomenclatural confusion [28]. The name C. zedoaria has been applied to several taxa in Asia that possess a purple band along the midvein on the upper surface of leaves [3, 19, 21]. The Japanese population of C. zedoaria has been cultivated as “Gajyutsu” (“Ezhu” in Chinese) and has been prescribed in the Japanese Pharmacopoeia since the third edition in the year 1906 [29]; however, the original source of Japanese C. zedoaria is unclear. Kitamura et al. reported that “Gajyutsu” cultivated in Yakushima Island, Japan, was more similar to C. aeruginosa than C. zedoaria from Java, Indonesia, based on trnK intron sequence, random amplified polymorphic DNA analysis and essential oil composition [30]. Our study based on morphology, ILP markers and trnK intron sequence clearly distinguished Japanese C. zedoaria from Indonesian C. aeruginosa. However, crude drug sample D25843 from Indonesia deduced to be C. zedoaria through its local name “Kunir putih,” showed the same ILP pattern as well as trnK intron sequence as the Japanese C. zedoaria, which suggested that Japanese C. zedoaria had a close relation to Indonesian C. zedoaria. However, Indonesian crude drug sample D24986 showed identical ILP patterns to Japanese C. zedoaria but the same trnK intron sequence as C. aeruginosa, suggesting possible hybridization between these two species in Indonesia. Plant specimen I-0005 from India identified as C. zedoaria also showed the same ILP pattern and trnK intron sequence as Japanese C. zedoaria, suggesting close relations of the C. zedoaria populations in Japan, Indonesia and India. However, a further study based on morphological and molecular analyses of widely collected specimens from India and Indonesia is needed to determine any relationship between Japanese C. zedoaria and C. zedoaria in India and Indonesia.
The group P, including six plant specimens and two crude drug samples of C. phaeocaulis from China, showed the Ptk type of trnK intron sequence. All plant specimens from China and one specimen identified as C. phaeocaulis from Thailand showed an identical ILP pattern, whereas the two crude drug samples showed minor differences from this.
The group Ae included plant specimens Q41 and Q47 originally introduced from China and 10167 of C. aeruginosa introduced from Indonesia, as well as crude drug sample D22069 from Thailand, which was deduced to be C. aeruginosa from its local name “Wan maha mek.” All of them had an identical ILP pattern. Although C. aeruginosa showed the same Ptk type of trnK intron sequence as C. phaeocaulis, the distinguishable ILP patterns allowed clear discrimination of these two species.
The group K included five plant specimens of C. kwangsiensis introduced from China, among which four had K(gl)Wtk type and one had Ltk(10T)2207T type of trnK intron sequence. Our previous study using field investigation and morphological, genetic, and chemical analyses suggested C. kwangsiensis was of hybrid origin [25]. The variant ILP patterns of all five C. kwangsiensis specimens also indicated its genetic diversity.
In group W, three plant specimens of C. wenyujin introduced from China had identical ILP patterns and the same K(gl)Wtk type of trnK intron sequence.
The group Za was composed of five plant specimens of C. zanthorrhiza from China, Japan, Malaysia, Indonesia and India, and eight crude drug samples produced in Indonesia, India and Thailand. These samples had an identical ILP pattern and the same K(pl)Ztk(6A14T) type of trnK intron sequence (Fig. 3). Among the eight crude drug samples, four samples with local name “Temu lawak” from Indonesia were deduced to be C. zanthorrhiza, which was supported by our genetic analysis data. The other three samples from India (D20477, D20483 and D22836), deduced to be C. aromatica through the local name “Kasturi manjal,” were included in the Za group. C. zanthorrhiza is indigenous to South India; however, it has long been misidentified as C. aromatica in India [21]. Our molecular analysis revealed that the botanic source of the crude drug “Kasturi manjal” in India was C. zanthorrhiza not C. aromatica. A similar situation applied to crude drug sample D24867 from Thailand; it was deduced to be C. aromatica due to its local name “Wan narn kum,” while its botanic source was C. zanthorrhiza.
The group A/M included three plant specimens and five crude drug samples from Indonesia, Thailand, Myanmar and India. Plant specimen 00959 of C. mangga from Thailand, plant specimen I-0007 of C. amada from India and crude drug sample D30515 from Myanmar had an identical ILP pattern. In Bangladesh, C. amada was first recorded in the 1810s and its fresh rhizome with a smell of green mango is a distinguishable character of this species [31]. In the 1910s, another Curcuma species with rhizomes smelling of green mango was recorded in Java, Indonesia, and named C. mangga. Although the main morphological difference between the two species has been reported as a central inflorescence in C. amada and a lateral inflorescence in C. mangga [32], some reports described that C. amada in India can have a lateral or central inflorescence [33, 34]. Our molecular analysis revealed that their ILP patterns were indistinguishable. In addition, plant specimen 00591 of C. amada from India and crude drug samples D30516 of C. amada from Myanmar and D24983 of C. mangga from Indonesia belonged to the same subclade with C. zanthorrhiza, group Za in the phylogenetic tree (Fig. 4).
The group Pe included one plant specimen of C. petiolata and uncertain plant specimen 94008. The two specimens showed Ltk(11T) type of trnK intron sequences, while specimen 94008 had an identical pattern in DCS intron I region and similar patterns in DCS intron II and CURS intron regions with the C. petiolata specimen. As described in the section “Morphological identification of plant specimens”, the specimen 94008 had characteristic leaves with a creamy white margin, similar to varieties of C. petiolata. Together with the molecular data, it is reasonable to conclude that this specimen was C. petiolata.
The group C included six crude drug samples from Thailand. In Thailand, the crude drug with the local name “Wan chak modluk” is generally considered to be C. comosa. The five “Wan chak modluk” samples showed different ILP patterns, among which four samples showed K(pl)Ztk(7A15T) type and one sample had the K(pl)Ztk(7A13T) type of trnK intron sequence. Crude drug sample D30522 with the name “Wan maha mek,” deduced to be C. aeruginosa, showed the same type of trnK intron sequence and similar ILP patterns to C. comosa. Therefore, we suspect the name of this sample is wrong.
The molecular information provided by ILP markers and trnK intron sequences was demonstrated to be useful for taxonomic arrangement of Asian Curcuma species and standardization of Asian Curcuma drugs. For obtaining more concise results on these difficult questions, however, further study including morphological comparison with the specimens from type locality and molecular investigation on variability of ILP pattern in hybrid plants is needed. Based on the present study, the botanical origins of “Khamin oi” and “Wan narn kum” from Thailand and “Kasturi manjal” from India are completely different from the general claims, suggesting these crude drugs should be used with caution.
Conclusion
In this study, to elucidate specific molecular markers of medicinally used Curcuma species in Asia, to solve confusion on the reported botanical origin of crude drugs and to locate the original habitats of C. aromatica and C. zedoaria cultivated in Japan, molecular analysis based on the ILP markers in DCS and CURS genes and the trnK intron sequences was performed using 59 plant specimens and 42 crude drug samples, which belonged to 13 Curcuma species obtained from Asian countries. The ILP patterns of the respective species revealed high consistency in C. aromatica, C. zedoaria, C. phaeocaulis, C. aeruginosa, C. wenyujin and C. zanthorrhiza, and showed intraspecies polymorphism in C. longa, C. kwangsiensis, C. amada, C. mangga and C. comosa. The C. longa specimens and samples were separated into three subgroups which were highly consistent with their geographical origins. Based on the ILP markers and the trnK intron sequences, the botanical origins of “Khamin oi” were correctly determined to be C. longa or a hybrid between C. longa and other species with a K(pl)Ztk(7A13T) type of trnK intron sequence, and “Wan narn kum” from Thailand and “Kasturi manjal” from India were correctly determined to be C. zanthorrhiza. Moreover, morphological and molecular data showed that C. aromatica and C. zedoaria cultivated in Japan had close relations with C. aromatica from China and Thailand, and C. zedoaria from Indonesia and India, respectively. Thus, ILP markers in DCS and CURS genes combined with the trnK intron sequences were demonstrated to be useful for the standardization of Asian Curcuma drugs.
Change history
29 March 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11418-023-01694-x
References
The Ministry of Health, Labour and Welfare (2016) The Japanese Pharmacopoeia XVII, p 2005, 2012
Chinese Pharmacopoeia Commission (2015) Pharmacopoeia of The People’s Republic of China. China Med Sci Press 1:146–148
Ravindran PN, Babu KN, Sivaraman K (2007) Turmeric: The genus Curcuma. CRC Press, pp 1–27, 409–436, 451–467
Komatsu K, Kita T (2015) Turmeric, medicinally-used Curcuma plants and Curcuma drugs in Asia. Foods Food Ingred J Jpn 220:298–308
Wang J, Wang H, Zhu R, Liu Q, Fei J, Wang S (2015) Anti-inflammatory activity of curcumin-loaded solid lipid nanoparticles in IL-1β transgenic mice subjected to the lipopolysaccharide-induced sepsis. Biomaterials 53:475–483. https://doi.org/10.1016/j.biomaterials.2015.02.116
Tapia E, Sánchez-Lozada LG, García-Niño WR, García E, Cerecedo A, García-Arroyo FE, Osorio H, Arellano A, Cristóbal-García M, Loredo ML, Molina-Jijón E, Hernández-Damián J, Negrette-Guzmán M, Zazueta C, Huerta-Yepez S, Reyes JL, Madero M, Pedraza-Chaverrí J (2014) Curcumin prevents maleate-induced nephrotoxicity: relation to hemodynamic alterations, oxidative stress, mitochondrial oxygen consumption and activity of respiratory complex I. Free Radic Res 48:1342–1354. https://doi.org/10.3109/10715762.2014.954109
Liu F, Gao S, Yang Y, Zhao X, Fan Y, Ma W, Yang D, Yang A, Yu Y (2018) Antitumor activity of curcumin by modulation of apoptosis and autophagy in human lung cancer A549 cells through inhibiting PI3K/Akt/mTOR pathway. Oncol Rep. https://doi.org/10.3892/or.2018.6188
Tohda C, Nakayama N, Hatanaka F, Komatsu K (2006) Comparison of anti-inflammatory activities of six Curcuma rhizomes: a possible curcuminoid-independent pathway mediated by Curcuma phaeocaulis extract. Evid-based complement. Altern Med ECAM 3:255–260. https://doi.org/10.1093/ecam/nel008
Tanaka K, Kuba Y, Ina A, Watanabe H, Komatsu K (2008) Prediction of cyclooxygenase inhibitory activity of Curcuma rhizome from chromatograms by multivariate analysis. Chem Pharm Bull (Tokyo) 56:936–940. https://doi.org/10.1248/cpb.56.936
Vinitha MR, Kumar US, Aishwarya K, Sabu M, Thomas G (2014) Prospects for discriminating Zingiberaceae species in India using DNA barcodes. J Integr Plant Biol 56:760–773. https://doi.org/10.1111/jipb.12189
Chen J, Zhao J, Erickson DL, Xia N, Kress WJ (2015) Testing DNA barcodes in closely related species of Curcuma (Zingiberaceae) from Myanmar and China. Mol Ecol Resour 15:337–348. https://doi.org/10.1111/1755-0998.12319
Minami M, Nishio K, Ajioka Y, Kyushima H, Shigeki K, Kinjo K, Yamada K, Nagai M, Satoh K, Sakurai Y (2009) Identification of Curcuma plants and curcumin content level by DNA polymorphisms in the trnS-trnfM intergenic spacer in chloroplast DNA. J Nat Med 63:75–79. https://doi.org/10.1007/s11418-008-0283-7
Záveská E, Fér T, Šída O, Krak K, Marhold K, Leong-Škorničková J (2012) Phylogeny of Curcuma (Zingiberaceae) based on plastid and nuclear sequences: proposal of the new subgenus Ecomata. Taxon 61:747–763. https://doi.org/10.1002/tax.614004
Cao H, Sasaki Y, Fushimi H, Komatsu K (2001) Molecular analysis of medicinally-used Chinese and Japanese Curcuma based on 18S rRNA gene and trnK gene sequences. Biol Pharm Bull 24:1389–1394. https://doi.org/10.1248/bpb.24.1389
Sasaki Y, Fushimi H, Cao H, Cai S-Q, Komatsu K (2002) Sequence analysis of Chinese and Japanese Curcuma drugs on the 18S rRNA gene and trnK gene and the application of amplification-refractory mutation system analysis for their authentication. Biol Pharm Bull 25:1593–1599. https://doi.org/10.1248/bpb.25.1593
Kita T, Komatsu K, Zhu S, Iida O, Sugimura K, Kawahara N, Taguchi H, Masamura N, Cai S-Q (2016) Development of intron length polymorphism markers in genes encoding diketide-CoA synthase and curcumin synthase for discriminating Curcuma species. Food Chem 194:1329–1336. https://doi.org/10.1016/j.foodchem.2015.08.034
Xiao X, Zhong G, Shu G, Li L, Fang Q, Chen S, Shu Z (2004) Numerical taxonomy of medicinal plants of Curcuma in China. China J Chin Mater Medica 29:15–24
Sirirugsa P, Larsen K, Maknoi C (2007) The genus Curcuma L. (Zingiberaceae): distribution and classification with reference to species diversity in Thailand. Gard Bull Singap 59:203–220
Jadhao AS, Bhuktar AS (2018) Genus Curcuma L. (Zingiberaceae) from Maharashtra State—India. Int J Curr Res Biosci Plant Biol 5:39–48
Wu Z, Raven PH, Hong D (2009) Flora of China. Science Press & Missouri Botanical Garden Press 24:359–361
Škorničková J, Sabu M (2005) The identity and distribution of Curcuma zanthorrhiza Roxb. (Zingiberaceae). Gard Bull Singap 57:199–210
Zhu S, Bai Y, Oya M, Tanaka K, Komatsu K, Maruyama T, Goda Y, Kawasaki T, Fujita M, Shibata T (2011) Genetic and chemical diversity of Eleutherococcus senticosus and molecular identification of Siberian ginseng by PCR-RFLP analysis based on chloroplast trnK intron sequence. Food Chem 129:1844–1850. https://doi.org/10.1016/j.foodchem.2011.05.128
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Komatsu K, Sasaki Y, Tanaka K, Kuba Y, Fushimi H, Cai S-Q (2008) Morphological, genetic, and chemical polymorphism of Curcuma kwangsiensis. J Nat Med 62:413–422. https://doi.org/10.1007/s11418-008-0272-x
Leong-Škorničková J, Šída O, Marhold K (2010) Back to types! Towards stability of names in Indian Curcuma L. (Zingiberaceae ). Taxon 59:269–282. https://doi.org/10.1002/tax.591025
Salisbury RA, Hooker W, Shury DN (1805) The paradisus londinensis:or coloured figures of plants cultivated in the vicinity of the metropolis. pp. Tab. 96, Explanation of Tab 96, Text https://www.biodiversitylibrary.org/page/36898363
Leong-Škorni J (2008) Taxonomic and nomenclatural puzzles in Indian Curcuma: the identity and nomenclatural history of C. zedoaria (Christm.) Roscoe and C. zerumbet Roxb (Zingiberaceae). Taxon 57:949–962
Satake M (2012) The Japanese Pharmacopoeia and its recorded crude drugs. Annu Rep Inst Nat Med Univ Toyama 38:2–13
Kitamura C, Nagoe T, Prana MS, Agusta A, Ohashi K, Shibuya H (2007) Comparison of Curcuma sp. in Yakushima with C. aeruginosa and C. zedoaria in Java by trnK gene sequence, RAPD pattern and essential oil component. J Nat Med 61:239–243
Asiatic Society of Bengal (1810) Asiatic researches, or, Transactions of the Society instituted in Bengal for inquiring into the history and antiquities, the arts, sciences and literature of Asia. Natural History Museum Library, London, p 341 https://www.biodiversitylibrary.org/page/42220931
Koninklijke Nederlandse Botanische Verenigingnische Vereniging (1904) Recueil des travaux botaniques néerlandais. Société botanique néerlandaise, Nimègue. 14: 127–132 https://www.biodiversitylibrary.org/page/15266684
Kumar R, Singh SK, Sharma S, Mao AA (2013) New and noteworthy records of gingers from north-east India. Keanean J Sci 2:13–18
Jatoi SA, Kikuchi A, Gilani SA, Watanabe KN (2007) Phytochemical, pharmacological and ethnobotanical studies in mango ginger (Curcuma amada Roxb.; Zingiberaceae). Phytother Res 21:507–516. https://doi.org/10.1002/ptr.2137
Acknowledgements
We greatly appreciate Assoc. Prof. Sitthithaworn Worapan at Srinakarinwirot University, Thailand, Prof. Emeritus Viswanathan M.V. at the University of Madras, Dr. Unnikrishnan Payyappallimana at United Nations University, India, and Department of Traditional Medicine, Ministry of Health and Sports, Myanmar for kind help in collecting crude drug samples, and Dr. Naoko Anjiki, Research Center for Medicinal Plant Resources, National Institutes of Biomedical Innovation, Health and Nutrition, Prof. Emeritus Koichiro Komai and Prof. Masanori Morimoto at Kindai University and Kyoto Botanical Gardens, Japan for providing plant specimens. This work was supported in part by the grant from the Yamazaki Spice Promotion Foundation and by JSPS KAKENHI Grant numbers JP14406030, JP21406004, JP15H05268 and JP18K06714.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to retrospective open access order.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Q., Zhu, S., Hayashi, S. et al. Discrimination of Curcuma species from Asia using intron length polymorphism markers in genes encoding diketide-CoA synthase and curcumin synthase. J Nat Med 76, 69–86 (2022). https://doi.org/10.1007/s11418-021-01558-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-021-01558-2