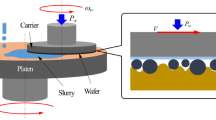
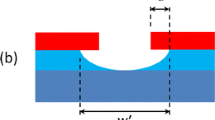
Abstract
Hydrogen peroxide (H2O2) plays a vital role in some of the metal assisted chemical etching (MACE) processes for silicon. However, it evaporates easily during etching at higher temperatures and this makes the process difficult to control. As a result, the MACE process for the inverted pyramid (IP) texturization that uses H2O2 is industrially unattractive. Herein, we proposed an innovative method to monitor H2O2 during the MACE process with an electrochemical method. The screen-printed electrode (SPE) modified by reduced graphene oxide (RGO) was used. The electrode demonstrated excellent electrochemical performance and could monitor the changes of H2O2 concentration with cyclic voltammetry (CV). Interestingly, the presence of copper (Cu) in the etching solution catalyzed not only the etching process, but also the electrochemical reduction of H2O2. With a consistent H2O2 concentration measured by the electrode, the reflectivity and structural morphology of the etched wafers could be controlled easily. The electrode is disposable, and the fabrication process is rapid and inexpensive, which is suitable for real time control of the MACE processes.
Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Baker-Finch SC, McIntosh KR (2011) Reflection of normally incident light from silicon solar cells with pyramidal texture. Prog Photovoltaics 19:406–416
Sun PY, Tsai PC, Liang PY, Hsu HP, Sutejo A, Yang A, Lan CW (2020) Green scalable vapor texture etching for multicrystalline silicon wafers. Prog Photovolt Res Appl. 1–8. https://doi.org/10.1002/pip.3302
Cao Y, Zhou Y, Liu F, Zhou Y, Zhang Y, Liu Y, Guo Y (2015) Progress and mechanism of Cu assisted chemical etching of silicon in a low Cu2+ concentration region. ECS J Solid State Sci Technol 4:331–336
Huang ZP, Geyer N, Liu LF, Li MY, Zhong P (2010) Metal-assisted electrochemical etching of silicon. Nanotechnol 21:465301
Wang Y, Liu Y, Yang L, Chen W, Du X, Kuznetsov A (2017) Micro-structured inverted pyramid texturization of Si inspired by self-assembled Cu nanoparticles. Nanoscale 9:907–914
Yang B, Lee M (2014) Mask-free fabrication of inverted-pyramid texture on single-crystalline Si wafer. Opt Laser Technol 63:120–124
Wang Y, Yang L, Liu Y, Mei Z, Chen W, Li J, Liang H, Kuznetsov A, Xiaolong D (2015) Maskless inverted pyramid texturization of silicon. Sci Rep 5:10843
Chen W, Liu Y, Wu J, Chen Q, Zhao Y, Wang Y, Du X (2019) High-efficient solar cells textured by Cu/Ag-cocatalyzed chemical etching on diamond wire sawing multicrystalline silicon. ACS Appl Mater Interfaces 11:10052–10058
Jiang B, Li M, Liang Y, Bai Y, Song D, Li Y, Luo J (2016) Etching anisotropy mechanisms lead to morphology-controlled silicon nanoporous structures by metal assisted chemical etching. Nanoscale 8:3085–3092
Liu Y, Lai T, Li H, Wang Y, Mei Z, Liang H, Li Z, Zhang F, Wang W, Kuznetsov AY, Du X (2012) Nanostructure formation and passivation of large-area black silicon for solar cell applications. Small 8:1392–1397
Yang Y, Sheng G, Li S, Yu J, Ma W, Qiu J, El Kolaly W (2020) Nano-Texturing of Silicon Wafers Via One-Step Copper-Assisted Chemical Etching. Silicon 12:231–238
Kubendhiran S, Sison G, Hsu HP, Lan CW (2020) Copper assisted inverted pyramids texturization of monocrystalline silicon in a nitrogen bubbling bath for highly efficient light trapping. Silicon :1–9
Liu T, Guo Y, Zhang Z, Miao Z, Zhang X, Su Z (2019) Fabrication of hollow CuO/PANI hybrid nanofibers for non-enzymatic electrochemical detection of H2O2 and glucose. Sens Actuators B Chem 286:370–376
Klassen NV, Marchington D, McGowan HC (1994) H2O2 determination by the I3-method and by KMnO4 titration. Anal Chem 66:2921–2925
Matsubara C, Kawamoto N, Takamura K (1992) Oxo [5, 10, 15, 20-tetra (4-pyridyl) porphyrinato] titanium (IV): an ultra-high sensitivity spectrophotometric reagent for hydrogen peroxide. Analyst 117:1781–1784
King DW, Cooper WJ, Rusak SA, Peake BM, Kiddle JJ, O’Sullivan DW, Melamed ML, Morgan CR, Theberge SM (2007) Flow injection analysis of H2O2 in natural waters using acridinium ester chemiluminescence: method development and optimization using a kinetic model. Anal Chem 79:4169–4176
Chang MC, Pralle A, Isacoff EY, Chang CJ (2004) A selective, cell-permeable optical probe for hydrogen peroxide in living cells. J Am Chem Soc 126:15392–15393
Zhou K, Zhu Y, Yang X, Luo J, Li C, Luan S (2010) A novel hydrogen peroxide biosensor based on Au–graphene–HRP–chitosan biocomposites. Electrochim Acta 55:3055–3060
Nandini S, Nalini S, Manjunatha R, Shanmugam S, Melo JS, Suresh GS (2013) Electrochemical biosensor for the selective determination of hydrogen peroxide based on the co-deposition of palladium, horseradish peroxidase on functionalized-graphene modified graphite electrode as composite. J Electroanal Chem 689:233–242
Jimenez-Perez R, Gonzalez-Rodriguez J, González-Sánchez MI, Gómez-Monedero B, Valero E (2019) Highly sensitive H2O2 sensor based on poly (azure A)-platinum nanoparticles deposited on activated screen printed carbon electrodes. Sens Actuators B Chem 298:126878
Zhu L, Guo X, Liu Y, Chen Z, Zhang W, Yin K, Li L, Zhang Y, Wang Z, Sun L, Zhao Y (2018) High-performance Cu nanoparticles/three-dimensional graphene/Ni foam hybrid for catalytic and sensing applications. Nanotechnol 29:145703
Rao CNR, Sood AK, Voggu R, Subrahmanyam KS (2010) Some novel attributes of graphene. J Phys Chem Lett 1:572–580
Hang T, Xiao S, Yang C, Li X, Guo C, He G, Li B, Yang C, Chen HJ, Liu F, Deng S (2019) Hierarchical graphene/nanorods-based H2O2 electrochemical sensor with self-cleaning and anti-biofouling properties. Sens Actuators B Chem 289:15–23
Bolotin KI, Sikes KJ, Jiang Z, Klima M, Fudenberg G, Hone J, Kim P, Stormer HL (2008) Ultrahigh electron mobility in suspended graphene. Solid State Commun 146:351–355
Raj MA, John SA (2013) Fabrication of electrochemically reduced graphene oxide films on glassy carbon electrode by self-assembly method and their electrocatalytic application. Phys Chem C 117:4326–4335
Kubendhiran S, Thirumalraj B, Chen SM, Karuppiah C (2018) Electrochemical co-preparation of cobalt sulfide/reduced graphene oxide composite for electrocatalytic activity and determination of H2O2 in biological samples. J Colloid Interface Sci 509:153–162
Thirumalraj B, Sakthivel R, Chen SM, Rajkumar C, Yu LK, Kubendhiran S (2019) A reliable electrochemical sensor for determination of H2O2 in biological samples using platinum nanoparticles supported graphite/gelatin hydrogel. Microchem J 146:673–678
Sakthinathan S, Kubendhiran S, Chen SM, Karuppiah C, Chiu TW (2017) Novel bifunctional electrocatalyst for ORR activity and methyl parathion detection based on reduced graphene oxide/palladium tetraphenylporphyrin nanocomposite. J Phys Chem C 121:14096–14107
Smith AW, Rohatgi A (1993) Ray tracing analysis of the inverted pyramid texturing geometry for high efficiency silicon solar cells. Sol Energy Mater Sol 29:37–49
Kudin KN, Ozbas B, Schniepp HC, Prud’Homme RK, Aksay IA, Car R (2008) Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett 8:36–41
Ferrari AC, Meyer JC, Scardaci V, Casiraghi C, Lazzeri M, Mauri F, Piscanec S, Jiang D, Novoselov KS, Roth S, Geim AK (2006) Raman spectrum of graphene and graphene layers. Phys Rev Lett 97:187401
Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y, Wu Y, Nguyen ST, Ruoff RS (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45:1558–1565
Das AK, Srivastav M, Layek RK, Uddin ME, Jung D, Kim NH, Lee JH (2014) Iodide-mediated room temperature reduction of graphene oxide: a rapid chemical route for the synthesis of a bifunctional electrocatalyst. J Mater Chem A 2:1332–1340
Yang J, Shen H, Jiang Y, Sun L (2019) Controllable fabrication and mechanism study of textured ultra-thin silicon wafers via one-step Cu-assisted chemical etching. Mater Sci Semicond Process 100:79–86
Deng SY, Zhang GY, Shan D, Liu YH, Wang K, Zhang XJ (2015) Pyrocatechol violet-assisted in situ growth of copper nanoparticles on carbon nanotubes: The synergic effect for electrochemical sensing of hydrogen peroxide. Electrochim Acta 155:78–84
Ensafi AA, Zandi-Atashbar N, Ghiaci M, Taghizadeh M, Rezaei B (2015) Synthesis of new copper nanoparticle-decorated anchored type ligands: applications as non-enzymatic electrochemical sensors for hydrogen peroxide. Mater Sci Eng C 47:290–297
Cheng C, Zhang C, Gao X, Zhuang Z, Du C, Chen W (2017) 3D network and 2D paper of reduced graphene oxide/Cu2O composite for electrochemical sensing of hydrogen peroxide. Anal Chem 90:1983–1991
Zhao S, Yuan G, Wang Q, Liu W, Zhang S, Liu Z, Wang J, Li J (2019) Morphology control of c-Si via facile copper-assisted chemical etching: Managements on etch end-points. Appl Surf Sci 489:776–785
Lu J, Zhang X, Liu N, Zhang X, Yu Z, Duan T (2016) Electrochemical detection of Cu2+ using graphene–SnS nanocomposite modified electrode. J Electroanal Chem 769:21–27
Acknowledgements
This work was sponsored by the Ministry of Science and Technology (MOST) and the United Renewal Energy Co. through the Advanced Green Energy Project.
Funding
This work was sponsored by the Ministry of Science and Technology (MOST) and the United Renewal Energy Co. through the Advanced Green Energy Project.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Subbiramaniyan Kubendhiran (SK), Gavin Sison and Hsiao Ping Hsu. The research idea was from Chung-Wen Lan (CWL) and the first draft was written by SK, and revised by CWL. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest/Competing Interests
The authors declare that they have no conflicts of interest.
Ethics Approval
The manuscript should not be submitted to more than one journal for simultaneous consideration.
Consent to Participate
All authors approved the final manuscript and agreed to submit it to the journal of Silicon.
Consent for Publication
All authors approved the final manuscript and agreed to submit it to the journal of Silicon.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lan, CW., Kubendhiran, S., Sison, G. et al. Monitoring Hydrogen Peroxide Using an Electrochemical Method During Metal Assisted Chemical Etching for Silicon. Silicon 14, 5673–5681 (2022). https://doi.org/10.1007/s12633-021-01348-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-021-01348-1